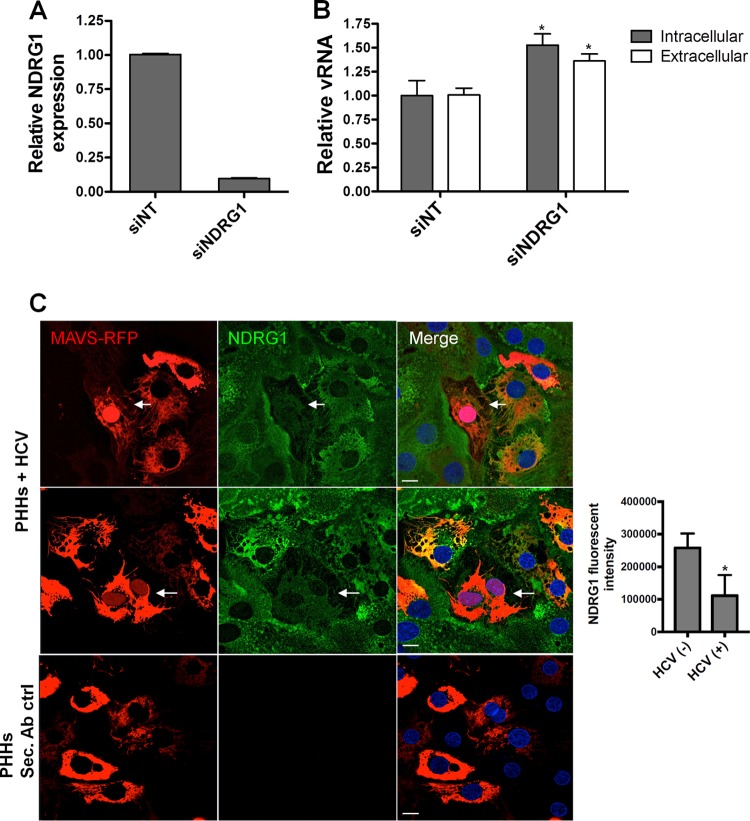
FIG 3.
NDRG1 knockdown alters HCV infection in primary human hepatocytes (PHHs). (A) Efficient knockdown of NDRG1 in PHHs. Knockdown was performed by transfecting PHHs with a pool of 4 NDRG1-specific siRNAs. Control cells were transfected with scrambled nontargeting siRNAs (siNT). At 72 h posttransfection, total RNA was harvested, and the NDRG1 mRNA level was measured by RT-qPCR. Expression was normalized to GAPDH mRNA detection. (B) HCV infection is enhanced in NDRG1-depleted PHHs. Knockdown was performed as described above for panel A, and both control and knockdown cells were infected with HCV. Total intracellular and extracellular RNAs were harvested 48 h later, and HCV RNA was quantified by RT-qPCR. (C) NDRG1 levels are reduced in HCV-infected PHHs. HCV-dependent fluorescence relocalization (HDFR) was used to detect HCV infection of PHHs. PHHs were transduced and incubated with MAVS-RFP reporter lentivirus for 24 h and then infected with HCV for 48 h. NDRG1 staining was detected by using an anti-NDRG1 antibody and anti-mouse Alexa Fluor 488. Cells with red nuclei indicate HCV-infected cells. Sec. Ab ctrl (secondary antibody control) indicates the absence of primary antibody incubation. Error bars denote standard deviations. All experiments were performed three times, and the data from a representative experiment are shown. **, P ≤ 0.01; *, P ≤ 0.05 (compared to the negative controls). Bars, 10 μm.