FIG 1.
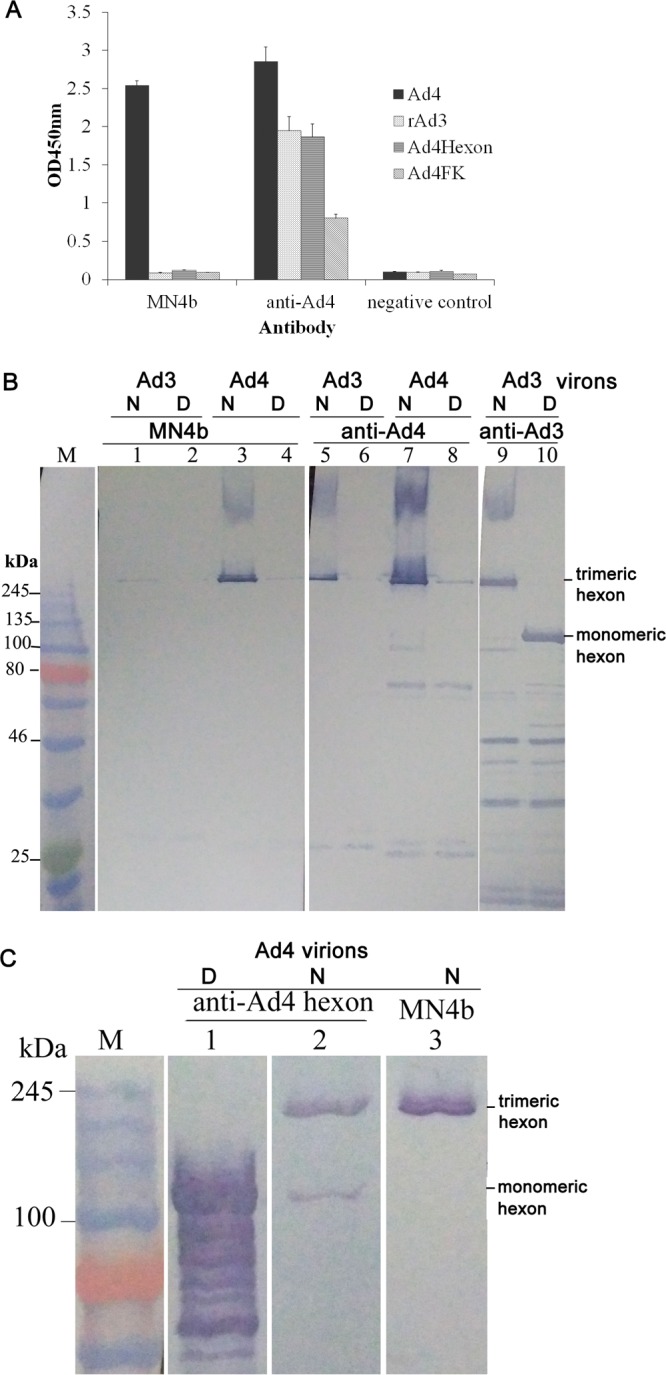
MAb MN4b recognizes HAdV-4 hexon in its trimeric form. (A) The reaction of MN4b with wild-type HAdV-4 (wAd4), rAd3, a recombinant hexon fragment (Ad4hexon), or fiber knob (Ad4FK) of HAdV-4 was detected by ELISAs. Antiserum from mice immunized with HAdV-4 (anti-Ad4) was used as the positive control, and antiserum from mice immunized with PBS was used as the negative control. Each experiment was repeated independently at least three times, and the means ± standard deviations are shown. OD450nm, optical density at 450 nm. (B) Immunoblot analysis indicates that MN4b recognizes HAdV-4 hexon in its trimeric form but not the hexon monomer. Purified HAdV-4 virions were stored at room temperature (native [N]) (lanes 3 and 7) or heated at 98°C (denatured [D]) (lanes 4 and 8) for 5 min in the presence of loading buffer. Purified HAdV-3 virions at room temperature (native) (lanes 1, 5, and 9) or boiled at 98°C (denatured) (lanes 2, 6, and 10) were used as the controls. The samples were then separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes were incubated with MN4b (lanes 1 to 4) or anti-Ad4 antiserum (lanes 5 to 8). Purified HAdV-3 virions that disintegrated at room temperature (lane 9) or at 98°C (lane 10) were incubated with mouse anti-HAdV-3 serum as controls. (C) Immunoblot analysis confirmed that MN4b recognizes HAdV-4 hexon. Purified HAdV-4 virions were stored at room temperature (native) (lanes 2 and 3) or heated at 98°C (denatured) (lane 1) for 5 min in the presence of loading buffer. The membranes were then incubated with MN4b (lane 3) or antiserum from a mouse immunized with recombinant HAdV-4 hexon expressed in E. coli (anti-Ad4hexon) (lanes 1 and 2). M, standard prestained protein marker (NEB, UK).
