Abstract
AIM
To evaluate the control, survival, and hepatic function for Child Pugh (CP)-A patients after Stereotactic body radiotherapy (SBRT) in hepatocellular carcinoma (HCC).
METHODS
From 2009 to 2016, 40 patients with Barcelona Liver Clinic (BCLC) stages 0-B HCC and CP-A cirrhosis completed liver SBRT. The mean prescription dose was 45 Gy (40 to 50 Gy in 4-5 fractions). Local relapse, defined as recurrence within the planning target volume was assessed with intravenous multiphase contrast computed tomography or magnetic resonance imaging every 4-6 mo after completion of SBRT. Progression of cirrhosis was evaluated by CP and Model for End Stage Liver Disease scores every 3-4 mo. Toxicities were graded per the Common Terminology Criteria for Adverse Events (v4.03). Median follow-up was 24 mo.
RESULTS
Forty-nine HCC lesions among 40 patients were analyzed in this IRB approved retrospective study. Median tumor diameter was 3.5 cm (1.5-8.9 cm). Six patients with tumors ≥ 5 cm completed planned selected transarterial chemoembolization (TACE) in combination with SBRT. Eight patients underwent orthotropic live transplant (OLT) with SBRT as a bridging treatment (median time to transplant was 12 mo, range 5 to 23 mo). The Pathologic complete response (PCR) rate in this group was 62.5%. The 2-year in-field local control was 98% (1 failure). Intrahepatic control was 82% and 62% at 1 and 2 years, respectively. Overall survival (OS) was 92% and 60% at 1 and 2 years, with a median survival of 41 mo per Kaplan Meier analysis. At 1 and 2 years, 71% and 61% of patients retained CPA status. Of the patients with intrahepatic failures, 58% developed progressive cirrhosis, compared to 27% with controlled disease (P = 0.06). Survival specific to hepatic failure was 92%, 81%, and 69% at 12, 18, and 24 mo. There was no grade 3 or higher toxicity. On univariate analysis, gross tumor volume (GTV) < 23 cc was associated with freedom from CP progression (P = 0.05), hepatic failure-specific survival (P = 0.02), and trended with OS (P = 0.10).
CONCLUSION
SBRT is safe and effective in HCC with early cirrhosis and may extend waiting time for transplant in patients who may not otherwise be immediate candidates.
Keywords: Stereotactic body radiotherapy, Hepatocellular carcinoma, Child-Pugh A, Cirrhosis, Hepatoma, Local control, Radiotherapy, Radiation
Core tip: This retrospective review demonstrates excellent long term local control of hepatocellular carcinoma (HCC) in early stage cirrhosis treated by Stereotactic body radiotherapy (SBRT), while retaining hepatic function. However, the overall prognosis of HCC remains poor despite successful local therapy and transplant remains the standard of care. Given the rising incidence of HCC, liver procurement and selection of candidates for transplant will become increasingly stringent. The long term control and maintenance of hepatic reserve demonstrated in this series suggests that SBRT as a bridging therapy may extend waiting time for transplant in patients who may not otherwise be immediate candidates for it.
INTRODUCTION
Accounting for the second most cancer-related deaths worldwide, hepatocellular carcinoma (HCC) is an aggressive malignancy that is diagnosed in at least 6 of every 100000 Americans, a rate nearly triple that of thirty years ago[1,2]. In the United States, Chronic Hepatitis C (HCV), alcohol abuse, and non-alcoholic steatohepatitis (NASH) are the leading causes of HCC, which is diagnosed at a growing rate in light of more sophisticated imaging and vigilant surveillance with serum markers[3-5]. Liver transplant remains the gold standard for definitive treatment, however the vast majority of patients fail to meet the surgical or medical criteria for transplant, with high mortality rates if not properly selected[6]. Further complicating management is the cirrhosis that accompanies HCC, which often renders patients medically inoperable or at high risk for surgery.
Therapeutic alternatives include partial hepatectomy, radiofrequency ablation, trans-arterial chemoembolization (TACE), and radioembolization among others. Each treatment modality is associated with procedural complications especially in patients with portal hypertension. In non-cirrhotic patients, partial hepatectomy or surgical resection of hepatocellular carcinoma is potentially curative, with average long-term intrahepatic control rates over 40% and 5-year survival over 60%[7,8]. However, cirrhotic patients must be carefully selected for partial resection to avoid access perioperative mortality[9,10]. Further limiting patient selection for resection are tumors with vascular invasion or those in a centralized location, even in otherwise healthy livers[11]. Ultimately, 15%-30% of HCC patients are eligible for curative partial hepatectomy[12,13]. Other widely used modalities such as TACE and RFA in non-surgical candidates have shown a control and survival benefit, however selection is limited by vascular invasion and biliary obstruction with TACE[14], and by size (< 3 cm) and location (infradiaphragmatic or adjacent to large vessels) with RFA[15,16].
Stereotactic body radiotherapy (SBRT) has emerged as non-invasive treatment that serves as another alternative for local tumor control or used as a bridge to liver transplant. SBRT by definition is an ultraconformal radiotherapy technique administering high radiotherapy doses in 1-5 fractions. It uses multiple external radiation beams/arcs deliver an ablative tumoricidal dose with sharp dose fall-off which limits unacceptable dose to the liver as well as adjacent vasculature, gallbladder, chest wall, kidney or diaphragm.
Several prospective studies have shown that SBRT can be delivered safely in Child Pugh A patients with local control rates between 75%-90% for median tumor size between 20 - 30 cc[17,18].
Although the data for SBRT in HCC is promising, current guidelines recommend it only when patients are not amenable to, or have failed, other local therapies. Furthermore, while a favorable short-term SBRT-related toxicity profile in early cirrhotic patients is well documented, its long-term impact on progression of hepatic failure is not widely reported. The objective of this retrospective study is to analyze the tumor control, survival, toxicity and preservation of hepatic function, in HCC patients with Child-Pugh A cirrhosis treated with SBRT.
MATERIALS AND METHODS
Patient selection
Between 2009 and 2016, 49 intrahepatic lesions among 40 patients with BCLC stages 0-B hepatocellular carcinoma and Child-Pugh class A cirrhosis were treated with SBRT at a single institution in this IRB approved study. Patients who were treated with palliative intent at a dose range below 30 Gy, had large multinodular tumors (aggregate > 9 cm), metastatic disease, or an ECOG performance status > 2 were excluded from this study. No patients had previous external beam radiation or Yttrium-90 radioembolization. Six patients with large tumors (median diameter 5.4 cm) received planned TACE prior to SBRT for radiosensitization. All patients were evaluated for hepatectomy and transplant in a multidisciplinary setting prior to undergoing SBRT.
Treatment
Treatment planning consisted of a IV contrast-enhanced free breathing helical computed tomography (CT) scan with 3 mm slice thickness, followed by immediate 4-D CT simulation utilizing a Siemens Somatom Sensation Open scanner (Siemens Medical) with an Anzai belt (AZ733V, Anzai Medical) and immobilization with a Vac-Loc® vacuum bag (Bionix, Toledo, OH, Spain). An internal target volume (ITV) was generated based on hepatic motion during the respiratory cycle, with a planning target volume (PTV) generated in the standard fashion around this volume. PTV included the ITV with a 0.3-0.5 cm margin. SBRT dose was prescribed to the isodose line encompassing the PTV (generally 80%-90% isodose line) allowing up to 20% higher dose to the target volume. Dose per fraction varied based on tumor size, location, and normal tissue tolerance. Twenty-two of the 38 patients utilized 4DCT co-registered with 99mTc-sulfur colloid Single Photon Emission Computed Tomography (SPECT) for visualization and conformal avoidance of best perfused hepatic parenchyma. Details of SPECT/CT co-registration and treatment planning have been previously reported for liver SBRT in cirrhotic HCC patients[19,20]. Dose limits were set such that at least 35% of predicted liver volume by SPECT imaging received ≤ 18 Gy in 5 fractions or ≤ 16 Gy in 4 fractions. The median dose to the PTV was 45 Gy (range 40 to 50 Gy) at a median dose per fraction of 9 Gy. Median biologic equivalent dose (BED10) was 85.5 Gy (range 72-105.6 Gy).
Outcome assessment
Local response with contrast-enhanced triple phase CT or MRI was documented every 4-6 mo following radiotherapy as per Response Evaluation Criteria in Solid Tumors (RECIST) criteria[21]. Failures were considered local if within or on the edge of the PTV. Intrahepatic failures were defined as radiographic evidence of progressive hepatocellular carcinoma within the liver and outside of the PTV. Fluctuations in alpha-feto protein (AFP) levels were not considered when assessing response or tumor control. The progression of cirrhosis was evaluated by Child-Pugh and End Stage Liver Disease (MELD) scores at least every 4 mo. Potential prognostic correlates including initial stage, tumor size, radiation dose, performance status, and initial MELD stage were analyzed against intrahepatic control, overall survival, and hepatic-failure specific survival, which we define as the portion of patients who did not die from liver failure. We also evaluated potential correlates of freedom from C-P progression, which we define as advancing from the Child Pugh A to the Child Pugh B classification[22]. Toxicities were graded per the Common Terminology Criteria for Adverse Events (CTCAE) (v4.03). Survival and tumor control analyses are based on Kaplan Meier (KM) methodology, and univariate analysis was conducted via Cox proportional hazard regression models using MedCalc.
RESULTS
Patient characteristics
Thirty-two males and eight females with HCC and CP-A cirrhosis who completed liver SBRT were analyzed with a median follow up of 24 mo (4 to 64 mo). Seven of the 40 patients had two tumors treated simultaneously, and one patient had 3 treated at the same time. The maximum tumor diameter ranged from 1.5 to 8.9 cm, with a median of 3.5 cm. Gross tumor volume varied between 2.6 to 220.1 cc with median 23 cc, and the corresponding planning target volume was between 11.5 and 351 cc (median 67.6). BCLC stages 0 (very early), A (early), and B (intermediate) comprised of 6, 10, and 24 patients, respectively. This corresponds to American Joint Committee on Cancer (AJCC) stages I (n = 6), II (n = 12), IIIA (n = 8) and IIIB (n = 8). SBRT was used as a bridging therapy for orthotropic liver transplant in eight patients. The causes of HCC include Hepatitis C (n = 17), alcohol abuse (n = 8), a combination of both (n = 8), NASH (n = 4), biliary cirrhosis (n = 1), immunosuppression following kidney transplant (n = 1), and one was cryptogenic. Eastern Cooperative Oncology Group (ECOG) performance status was equal to 0, 1, and 2 in 21, 14, and 3 patients respectively (2 unknown). Although all patients were classified as Child Pugh A, 9 of the 40 patients had a MELD score of 10 or higher. A summary of patient characteristics is demonstrated on Table 1.
Table 1.
Patient characteristics
| Number | Percentage | |
| Gender | ||
| Male | 32 | 82% |
| Female | 8 | 18% |
| ECOG performance status1 | ||
| 0 | 21 | 55% |
| 1 | 14 | 37% |
| 2 | 3 | 8% |
| Etiology of hepatocellular carcinoma2 | ||
| Hepatitis C | 17 | 46% |
| Alcohol | 8 | 22% |
| Combination of Hepatitis C/alcohol | 8 | 22% |
| NASH | 4 | 8% |
| BCLC Stage | ||
| 0 (very early) | 6 | 15% |
| A (early) | 10 | 25% |
| B (intermediate) | 24 | 60% |
| Previous treatment | ||
| None | 34 | 85% |
| TACE | 6 | 15% |
| Number of treated lesions | ||
| Single | 32 | 80% |
| Multiple3 | 8 | 20% |
| Initial MELD score | ||
| < 10 | 31 | 78% |
| > 10 | 9 | 22% |
| Median tumor size (range) | 3.5 cm | (1.5 to 8.9 cm) |
| Median gross tumor volume (range) | 23 cc | (2.6 to 220.1 cc) |
| Median planning target volume (range) | 67.6 cc | (11.5 to 351 cc) |
2 patients unknown;
1 patient with biliary cirrhosis and 1 immunosuppressed;
7 patients with 2 lesions and 1 with 3 lesions. ECOG: Eastern cooperative oncology group; NASH: Non-alcoholic steatohepatitis; BCLC: Barcelona liver clinic; TACE: Transarterial chemo-embolization; MELD: Model for end stage liver disease.
Control
At last follow up, 48 of 49 lesions (98%) were controlled locally (within the PTV). The one failure was a 4.3 cm tumor with a GTV of 80 cc treated to 4500 cGy in 5 fractions. The recurrence occurred 10 mo after completing SBRT. Intrahepatic control, defined as no evidence of disease within the entire liver was 82%, 77%, and 62% at 12, 18, and 24 mo, respectively, with a median time to progression of 47 mo per KM analysis. Five of the intrahepatic failures were treated with additional SBRT and five were salvaged with either TACE (1), Y-90 (2), or resection (1). Distant metastases occurred in the peritoneum, bone, and lungs among 6 patients. SBRT served as bridge for orthotropic liver transplant in 8 patients, 5 of whom demonstrated a pathologic complete response (62.5%). The median time to transplant was 12 mo (5-23 mo). One patient developed an intrahepatic failure which was successfully treated with a second SBRT prior to transplant. No patient developed recurrence after transplant.
Survival
Twenty-three of 40 (58%) patients were alive at last follow up. Three patients died from perioperative complications after liver transplant, all of whom retained Child Pugh A status and had a pathologic complete response. The remaining 5 transplant patients were all long term survivors. One (89% vs 88%) and two-year survival (60% vs 63%) was similar for patients who received SBRT with or without transplant. Progressive HCC was the cause of death in 9 patients treated with SBRT, and five patients died without evidence of recurrence, 3 of whom had progressive cirrhosis, one with heart disease, and one with metastatic lung cancer.
The median survival was 41 mo with a 1-year, 18-month, and 2-year overall survival rate of 92%, 74%, and 60%, respectively. Disease-free survival was 79%, 58%, and 44% at 1 year, 18 mo, and 2 years. Hepatic failure-specific survival was 92%, 81%, and 69% at 1 year, 18 mo, and 2 years, respectively. Univariate analysis suggested that a GTV > 23 cc correlated with a decreased hepatic failure-free survival (HR = 5.72, P = 0.01) and trended towards a decreased overall survival (HR = 2.14, P = 0.10). Advancing Child Pugh cirrhosis also strongly correlated with survival (HR 5.05, P = 0.01) (Figure 1, Figure 2, Figure 3).
Figure 1.
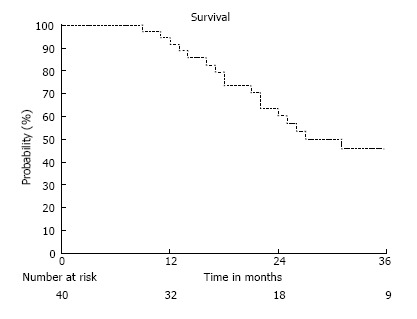
Overall Survival of all patients.
Figure 2.
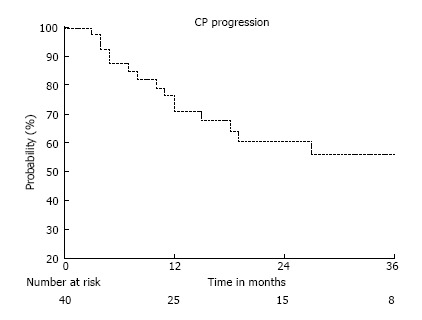
Freedom from child Pugh Progression of all patients. CP progression: Percentage of patients retaining child Pugh A status.
Figure 3.
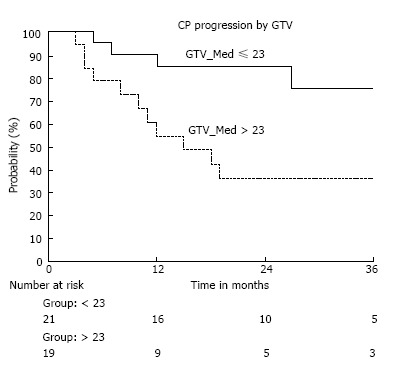
Freedom from child Pugh progression by gross tumor volume. CP progression: Percentage of patients retaining child Pugh A status; GTV: Gross tumor volume in cubic centimeters; Group ≤ 23: Number of patients with a GTV less than or equal to 23 cc; Group > 23: Number of patients with a GTV greater than 23 cc.
Hepatic function and toxicity
Of the 40 patients treated, 24 retained Child Pugh A class cirrhosis (63%) and 27 maintained their initial MELD score (68%) at the time of last follow up. The median time to progression within Child Pugh category was 37 mo, with a freedom from Child Pugh progression rate of 89%, 71%, and 62% at 6, 12, and 18 mo respectively (Figure 2). The median time to progression of MELD score was 33 mo with a freedom from MELD progression rate of 95%, 88%, and 79% at 6, 12, and 18 mo respectively. Of the patients with intrahepatic failures, 58% also developed progressive cirrhosis, compared to 27% whom were regionally controlled (HR = 3.8, P = 0.06). As with survival, a GTV > 23 cc (median 60 cc, up to 220 cc) correlated with an increased rate of Child Pugh progression (HR = 2.89, P = 0.05) (Figure 3). There was no incidence of grade 3 or higher toxicity, and 3 patients had grade 2 fatigue. Grade 1 elevation in transaminases was seen in 9 patients, and 1 patient developed grade 2 rise in Alkaline Phosphatase, without any incidence of radiation induced liver disease (RILD).
DISCUSSION
Until recently, radiotherapy has been only infrequently used in targeting hepatocellular carcinoma because of the low tolerance of the whole liver to radiation and challenges associated with underlying liver dysfunction. Conversely, dose escalation studies at the University of Michigan with CT-based 3D-conformal radiotherapy planning established a correlation between the irradiated liver volume, the dose delivered, and the risk of radiation-induced liver disease[23]. The liver is a parallel organ and small volumes of liver can tolerate high doses of radiation when the whole liver mean dose can be minimized with techniques such as SBRT. As a result, several prospective SBRT studies have established a dose-response relationship in HCC with early stage cirrhosis, without compromising safety.
Mendez-Romero et al[17] and Tse et al[24] demonstrated long term local control rates of 75% and 65% with a median dose of 5 Gy x 5 fractions and 6 Gy x 6 fractions, respectively. Dose escalation to 48 Gy in 3 fractions yielded an 87% local control rate for CPA patients in a phase I/II study by Lasley et al[25] Similarly, Bujold et al[33] found that doses over 30 Gy (in 6 fractions) improved local control rates. Building on these and other data, the patients in our study were treated to a median BED10 of 85.5 Gy (45 Gy in 5 fractions). The 98% local control rate in this study compares favorably to already excellent historical controls, and the overall survival falls within the wide range of reported outcomes in the current literature (Table 2).
Table 2.
Summary of prospective stereotactic body radiotherapy studies in hepatocellular carcinoma patients with Child Pugh-A cirrhosis
| Study | No of lesions | Median dose-fractionation | Median GTV (cc) | Local control | Overall survival | Grade 3+ toxicity | Median follow-up (m) |
| Mendez-Romero et al[17], 2006 | 111 | 5 Gy × 5 | 22.3 | 75% | 75%, 40% | 36% | 12.9 |
| (22 mo) | (1, 2 yr) | ||||||
| Tse et al[24], 2008 | 21 | 6 Gy × 6 | 173 | 65% | 48% | 12% | 17.6 |
| (1 yr) | (1 yr) | ||||||
| Lasley et al[25], 2012 | 39 | 16 Gy × 3 | - | 91% | 72% | 4.60% | 33.3 |
| (2 yr) | (2 yr) | ||||||
| Bujold et al[33], 2013 | 102 | 6 Gy × 6 | 117 | 87% | 55%, 34% | 2% | 31 |
| (1 yr) | (1 yr, 2 yr) | ||||||
| Current study | 47 | 9 Gy × 5 | 23 | 98% | 92%, 60% | None | 24 |
| (2 yr) | (1 yr, 2 yr) |
Study includes Child Pugh B patients. GTV: Gross tumor volume; cc: Cubic centimeters; Gy: Gray.
In this report of CP-A patients with limited HCC treated with SBRT, 1 and 2 year survival was similar for patients with and without transplant. Given the inherent perioperative mortality risk of liver transplantation, these well selected early CP-A cirrhotic patients with limited extent of HCC may benefit from watchful waiting, reserving orthotopic liver transplantation at the time of further intrahepatic progression or following their natural cirrhosis progression to higher MELD scores. Such a preposition has been suggested by Merion and Wedd et al[26,27] whose large retrospective studies independently reported no detriment in survival when delaying transplant in very early stage cirrhosis. Accordingly, close follow-up and careful selection is essential with a watchful waiting approach. Additionally, with 2 year follow up survival is similar with or without transplant, yet long term cure of both HCC and cirrhosis with transplant, may yield a separation of survival curves with longer follow up.
Among the most important aspects of patient selection in HCC is the risk stratification based on hepatic function, such as the Child-Pugh or Model for End Stage Liver Disease (MELD), as patients with worse baseline cirrhosis are at higher risk for therapeutic toxicity. Teh and Cucchetti et al[28,29] have shown that a MELD score over 9 preceding partial liver resection is associated with increased perioperative mortality and decreased survival Other studies corroborate a link between initial MELD or Child Pugh score and survival in hepatocellular carcinoma[21,27]. Even in early stage cirrhosis, HCC has been known to accelerate the natural progression of liver failure, which can be impacted regardless of its initial severity[30]. It has also been suggested that a linear progression of liver failure, or serial trend in increasing MELD score, is a better predictor of outcome compared to initial MELD score[31]. These data underline the importance of preserving hepatic function while treating the malignancy that exacerbates it, even at an early stage.
Unsurprisingly, in this study, intrahepatic failure correlated strongly with progressive liver disease, which consequently correlated with overall mortality. Among patients treated with SBRT with controlled disease in the liver, 73% retained long term hepatic function which compares favorably to the natural progression of cirrhosis[32]. Three patients advanced to Child Pugh B cirrhosis within 6 mo of SBRT, none of whom had radiographic evidence of HCC. There was no evidence of classic RILD or radiation-induced grade 2 or higher toxicity.
This retrospective review demonstrates excellent long term local control of HCC in early stage cirrhosis treated by SBRT, while retaining hepatic function at a rate similar to historical norms. Unfortunately, the overall prognosis of HCC remains poor despite successful local therapy. Liver transplant remains the standard of care for definitive management. However, with the rising incidence of HCC, demand for healthy livers may outpace supply, and consequently, the selection of appropriate candidates for transplant will become more stringent. The long term local control and maintenance of hepatic reserve demonstrated in this series suggests that SBRT as a bridging therapy may extend waiting time for transplant in patients who may not otherwise be immediate candidates for it, such as those with Child-Pugh A cirrhosis and early stage HCC.
ARTICLE HIGHLIGHTS
Research background
Hepatocellular carcinoma (HCC) is an aggressive malignancy that is diagnosed in at least 6 of every 100000 Americans, a rate nearly triple that of thirty years ago. Liver transplant remains the gold standard for definitive treatment, however many patients fail to meet the surgical or medical criteria for transplant, with high mortality rates if not properly selected. Stereotactic body radiotherapy (SBRT) has emerged as non-invasive treatment option for HCC to achieve local tumor control and may be used as a bridge to liver transplant. Multiple external radiation beams/arcs delivered ablative doses with sharp dose fall-off at surrounding normal tissues allowing SBRT to be administered without limitations of unacceptable toxicity to the liver and adjacent vasculature, gallbladder, chest wall, kidney or diaphragm. Several prospective studies have shown that SBRT can be delivered safely in Child Pugh A patients with local control rates between 75%-90%.
Research motivations
Although the data for SBRT in HCC is promising, current guidelines recommend it only when patients are not amenable to, or have failed, other local therapies. Furthermore, while short-term SBRT-related toxicity in early cirrhotic patients is well documented, its long-term impact on hepatic failure progression is not widely reported.
Research objectives
The objective of this retrospective study is to analyze the tumor control, survival, toxicity and preservation of hepatic function, in HCC patients with Child-Pugh A cirrhosis treated with SBRT.
Research methods
We retrospectively reviewed 40 patients with Barcelona Liver Clinic (BCLC) stages 0-B HCC and CP-A cirrhosis completed liver SBRT from 2009-2016. Local relapse, defined as recurrence within the planning target volume was assessed with intravenous multiphase contrast CT or MRI every 4-6 mo after completion of SBRT. Progression of cirrhosis was evaluated by CP and Model for End Stage Liver Disease (MELD) scores every 3-4 mo. Toxicities were graded per the Common Terminology Criteria for Adverse Events (v4.03). Median follow-up was 24 mo.
Research results
The 2-year in-field local control was 98% (1 failure). Intrahepatic control was 82% and 62% at 1 and 2 years, respectively. Overall survival (OS) was 92% and 60% at 1 and 2 years, with a median survival of 41 mo. At 1 and 2 years, 71% and 61% of patients retained CPA status. Of the patients with intrahepatic failures, 58% developed progressive cirrhosis, compared to 27% with controlled disease (P = 0.06). Survival specific to hepatic failure was 92%, 81%, and 69% at 12, 18, and 24 mo. There was no grade 3 or higher toxicity. On univariate analysis, gross tumor volume (GTV) < 23 cc was associated with freedom from CP progression (P = 0.05), hepatic failure-specific survival (P = 0.02), and trended with OS (P = 0.10). Eight patients underwent orthotropic live transplant (OLT) with SBRT as a bridging treatment (median time to transplant was 12 mo, range 5 to 23 mo). The Pathologic complete response (PCR) rate in this group was 62.5%.
Research conclusions
This retrospective review demonstrates excellent long term local control of HCC in early stage cirrhosis treated by SBRT, while retaining hepatic function. However, the overall prognosis of HCC remains poor despite successful local therapy and transplant remains the standard of care. Given the rising incidence of HCC, liver procurement and selection of candidates for transplant will become increasingly stringent. The long term control and maintenance of hepatic reserve demonstrated in this series suggests that SBRT as a bridging therapy may extend waiting time for transplant in patients who may not otherwise be immediate candidates for it.
Research perspectives
Further prospective studies utilizing SBRT for HCC as a bridge to transplant are warranted.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Allegheny Health Network Institutional Review Board.
Informed consent statement: Informed consent was not required for this retrospective study as the analysis used anonymous clinical data obtained retrospectively after each patient agreed to treatment by written consent. Permission for waiver of consent was obtained through by the Allegheny Health Network Institutional Review Board.
Conflict-of-interest statement: We have no financial relationships to disclose.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D,D
Grade E (Poor): 0
Peer-review started: October 12, 2017
First decision: November 7, 2017
Article in press: December 5, 2017
P- Reviewer: Bramhall S, Mizuguchi T, Niu ZS, Tarantino G S- Editor: Cui LJ L- Editor: A E- Editor: Lu YJ
Contributor Information
Shaakir Hasan, Division of Radiation Oncology, Allegheny General Hospital Cancer Institute, Pittsburgh, PA 15212, United States. shaakir.hasan@ahn.org.
Ngoc Thai, Division of Radiation Oncology, Allegheny General Hospital Cancer Institute, Pittsburgh, PA 15212, United States; Division of Transplant Surgery, Allegheny General Hospital Cancer Institute, Pittsburgh, PA 15212, United States.
Tadahiro Uemura, Division of Radiation Oncology, Allegheny General Hospital Cancer Institute, Pittsburgh, PA 15212, United States; Division of Transplant Surgery, Allegheny General Hospital Cancer Institute, Pittsburgh, PA 15212, United States.
Vijay Kudithipudi, Division of Radiation Oncology, Allegheny General Hospital Cancer Institute, Pittsburgh, PA 15212, United States.
Paul Renz, Division of Radiation Oncology, Allegheny General Hospital Cancer Institute, Pittsburgh, PA 15212, United States.
Stephen Abel, Division of Radiation Oncology, Allegheny General Hospital Cancer Institute, Pittsburgh, PA 15212, United States.
Alexander V Kirichenko, Division of Radiation Oncology, Allegheny General Hospital Cancer Institute, Pittsburgh, PA 15212, United States.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60:1767–1775. doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 4.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;110:726–734; discussion 734-735. [PubMed] [Google Scholar]
- 7.Chen HY, Juan CC, Ker CG. Laparoscopic liver surgery for patients with hepatocellular carcinoma. Ann Surg Oncol. 2008;15:800–806. doi: 10.1245/s10434-007-9749-1. [DOI] [PubMed] [Google Scholar]
- 8.Yin Z, Fan X, Ye H, Yin D, Wang J. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1203–1215. doi: 10.1245/s10434-012-2705-8. [DOI] [PubMed] [Google Scholar]
- 9.Hackl C, Schlitt HJ, Renner P, Lang SA. Liver surgery in cirrhosis and portal hypertension. World J Gastroenterol. 2016;22:2725–2735. doi: 10.3748/wjg.v22.i9.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucchetti A, Cescon M, Trevisani F, Pinna AD. Current concepts in hepatic resection for hepatocellular carcinoma in cirrhotic patients. World J Gastroenterol. 2012;18:6398–6408. doi: 10.3748/wjg.v18.i44.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Citterio D, Facciorusso A, Sposito C, Rota R, Bhoori S, Mazzaferro V. Hierarchic Interaction of Factors Associated With Liver Decompensation After Resection for Hepatocellular Carcinoma. JAMA Surg. 2016;151:846–853. doi: 10.1001/jamasurg.2016.1121. [DOI] [PubMed] [Google Scholar]
- 12.Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245:909–922. doi: 10.1097/01.sla.0000254368.65878.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl. 2004;10:S46–S52. doi: 10.1002/lt.20044. [DOI] [PubMed] [Google Scholar]
- 14.Cho YK, Chung JW, Kim JK, Ahn YS, Kim MY, Park YO, Kim WT, Byun JH. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer. 2008;112:352–361. doi: 10.1002/cncr.23185. [DOI] [PubMed] [Google Scholar]
- 15.Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, Liu GJ, Liang JY, Lau WY. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115:1914–1923. doi: 10.1002/cncr.24196. [DOI] [PubMed] [Google Scholar]
- 16.Head HW, Dodd GD 3rd, Dalrymple NC, Prasad SR, El-Merhi FM, Freckleton MW, Hubbard LG. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology. 2007;243:877–884. doi: 10.1148/radiol.2433060157. [DOI] [PubMed] [Google Scholar]
- 17.Méndez-Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, Nuyttens JJ, Brandwijk RP, Verhoef C, Ijzermans JN, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. 2006;45:831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 18.Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K, Deluca J, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 19.Gayou O, Day E, Mohammadi S, Kirichenko A. A method for registration of single photon emission computed tomography (SPECT) and computed tomography (CT) images for liver stereotactic radiotherapy (SRT) Med Phys. 2012;39:7398–7401. doi: 10.1118/1.4766877. [DOI] [PubMed] [Google Scholar]
- 20.Kirichenko A, Gayou O, Parda D, Kudithipudi V, Tom K, Khan A, Abrams P, Szramowski M, Oliva J, Monga D, et al. Stereotactic body radiotherapy (SBRT) with or without surgery for primary and metastatic liver tumors. HPB (Oxford) 2016;18:88–97. doi: 10.1016/j.hpb.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 22.Tarantino G, Citro V, Conca P, Riccio A, Tarantino M, Capone D, Cirillo M, Lobello R, Iaccarino V. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol. 2009;9:89. doi: 10.1186/1471-230X-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Ten Haken RK, Knol J, Dawson LA, Pan C, Lawrence TS. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 24.Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, Sherman M, Dawson LA. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 25.Lasley FD, Mannina EM, Johnson CS, Perkins SM, Althouse S, Maluccio M, Kwo P, Cárdenes H. Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5:e443–e449. doi: 10.1016/j.prro.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 27.Wedd J, Bambha KM, Stotts M, Laskey H, Colmenero J, Gralla J, Biggins SW. Stage of cirrhosis predicts the risk of liver-related death in patients with low Model for End-Stage Liver Disease scores and cirrhosis awaiting liver transplantation. Liver Transpl. 2014;20:1193–1201. doi: 10.1002/lt.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, Cha S, Kamath P, Kim R, Nagorney DM. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207–1215; discussion 1215. doi: 10.1016/j.gassur.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G, Zanello M, Grazi GL, Pinna AD. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966–971. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- 30.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Merion RM, Wolfe RA, Dykstra DM, Leichtman AB, Gillespie B, Held PJ. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl. 2003;9:12–18. doi: 10.1053/jlts.2003.50009. [DOI] [PubMed] [Google Scholar]
- 32.Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 33.Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]


