Figure EV3. AlkCα proteins contain an insertion that may stabilize DNA bend in the absence of Ig‐like domain.
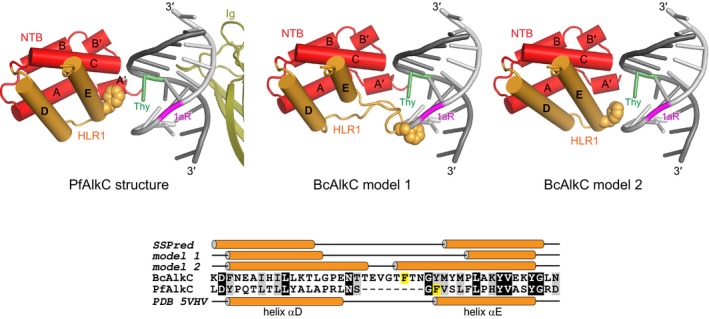
Two homology models for BcAlkC (center and right) are shown superimposed against the 1aR‐DNA from the PfAlkC/1aR‐DNA crystal structure (left). Only the N‐terminal helical bundle (NTB, red), HEAT‐like repeat 1 (HLR1, orange), and Ig‐like domain (olive) are shown for clarity. A phenylalanine side chain at the N‐terminus of helix αE in PfAlkC and in the 8‐residue insertion of BcAlkC is shown in spheres and highlighted yellow in the sequence alignment at the bottom. The secondary structural elements from the three models and from a secondary structure prediction of BcAlkC are shown against the sequences for each protein. The homology models were generated in SWISS‐MODEL (https://swissmodel.expasy.org/) using either the sequence alignment shown in Appendix Fig S1 (model 1) or generated by SWISS‐MODEL (model 2). In both models, the insertion makes contacts to the 1aR strand, either as a loop (model 1) or as an N‐terminal extension to helix αE (model 2).
