Figure 4. AlkC inserts its active site into the DNA .
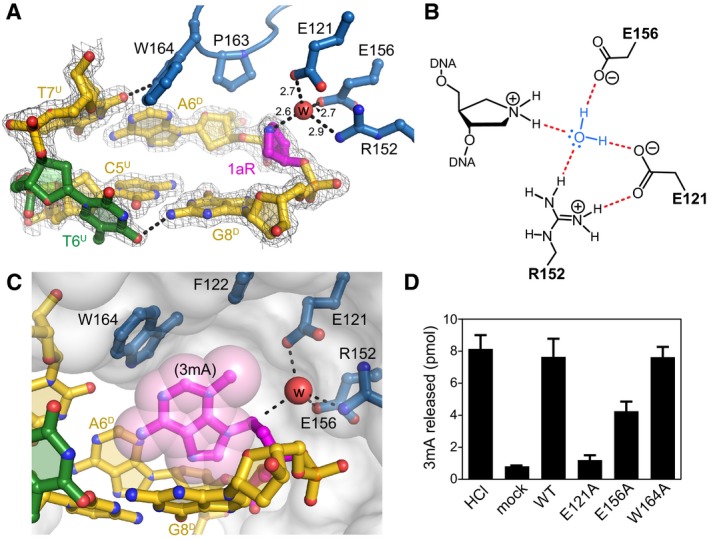
- Close‐up view of the AlkC active site (blue) bound to 1aR‐DNA (gold). The 1aR and opposite thymine are magenta and green, respectively. Water is shown as a red sphere, and hydrogen bonds are depicted as dashed lines. Composite omit electron density contoured to 1σ is superimposed against only the DNA for clarity. Superscripts in nucleotide labels refer to the 1aR‐containing, damaged (D) strand or the opposite, undamaged (U) strand. The pentaerythritol propoxylate molecule that occupies the active site has been omitted for clarity (see Fig EV4B).
- Schematic of the alignment of a catalytic water molecule (blue) against the 1aR oxocarbenium mimetic by AlkC active site residues. Hydrogen bonds are shown in red.
- A hypothetical model for AlkC bound to a 3mA‐DNA substrate was generated by superimposing the 3mA deoxyribose ring onto that of 1aR in the crystal structure, followed by rotating about the 3mA χ (N‐glycosidic bond) torsion angle to maximize van der Waals interactions. The solvent accessible surface of AlkC is shown as a transparent white envelope.
- Release of 3mA from methylated genomic DNA after a 5‐min incubation with either HCl, no enzyme (mock), PfAlkC (WT), E121A, E156A, or W164A. Values are mean ± SD (n = 3).
