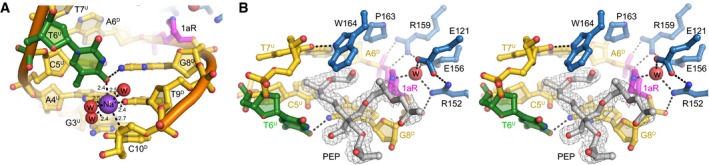
Figure EV4. Crystallographic features of the active site.
- Na+ ion coordination in the 1aR‐DNA structure. Na+ and water oxygens are shown as purple and red spheres, respectively. 1aR is magenta, and the opposite thymidine T6 is green. Superscripts in nucleotide labels refer to the 1aR‐containing, damaged (D) strand or the opposite, undamaged (U) strand. Hydrogen bonds are shown as dashed lines with interatomic distances in Ångstroms.
- Stereo‐views of the PfAlkC/1aR‐DNA active site. Active site residues are blue, and DNA is gold/magenta/green. Pentaerythritol propoxylate (PEP) sequestered from the solvent is in silver and superimposed against composite omit electron density contoured at 1σ. The putative catalytic water is shown as a red sphere. One arm of the PEP projects into the DNA kink between the 1aR and the flanking base pairs and thus may limit rotation of the 1aR ring back toward the DNA. The other two polymeric PEP arms project outward to solvent. The fourth PEP arm is not present; its terminal hydroxyl group forms a hydrogen bond to the displaced T6U thymine base. The position of the thymine is thus affected by the presence of the PEP in addition to its coordination by the Na+ ion. However, even in the absence of these stabilizing contacts, the T6U thymidine would be displaced into the minor groove as a result of the kink in the DNA. The space occupied by the PEP molecule would be occupied by the excised base both before and immediately after cleavage.
