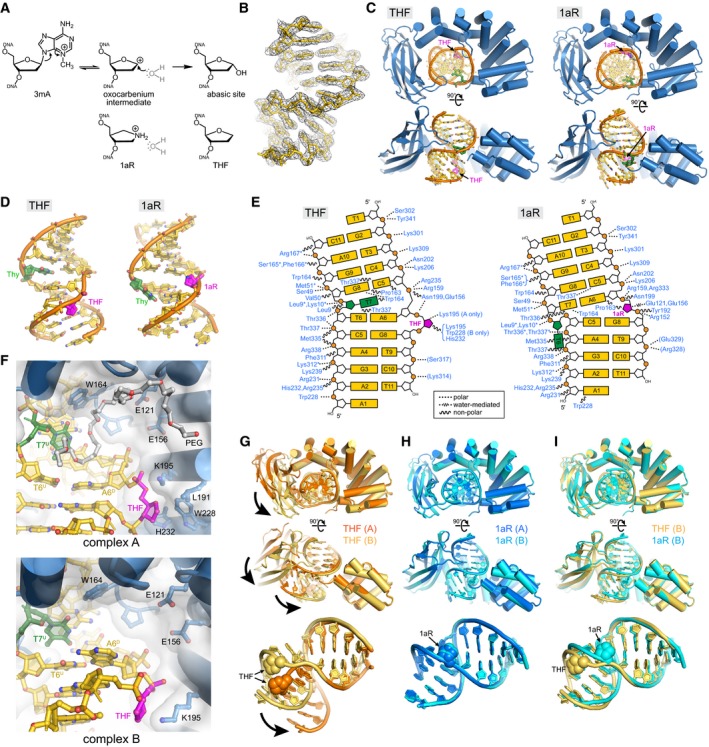
DNA glycosylase‐catalyzed reaction together with chemical structures of intermediate and product mimetics used in this study.
Annealed composite omit electron density contoured to 1σ is superposed onto the THF‐DNA crystallographic model.
Two views of the crystal structures, with protein in blue, DNA in gold, THF/1aR in magenta, and the thymidine opposite THF/1aR in green.
DNA structures extracted from the complexes.
Schematic of protein–DNA contacts. Contacts to the protein backbone are marked with an asterisk, and symmetry‐related contacts are in parentheses. PfAlkC maintains similar contacts with the DNA in THF and 1aR complexes relative to the position of the DNA bend and not to the position of the 1aR/THF.
Details of THF‐DNA bound outside of the PfAlkC active site. Both complexes in the crystallographic asymmetric unit are shown. A PEG 4,000 molecule (white carbons) fills the void in the catalytic pocket in complex A.
Superposition of the two protomers in the THF complex. Bold arrows highlight the difference in positions of the Ig‐like domain relative to the HLR domain and in the two DNA conformations.
Superposition of the two 1aR complexes.
Superposition of one protomer from each of the THF (gold) and 1aR (cyan) complexes.