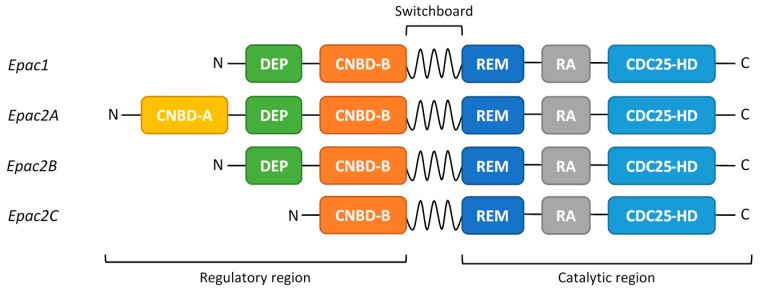
Figure 1.
Primary structure of the different exchange proteins activated by cyclic AMP (EPAC) isoforms. The N-terminal regulatory region is directly connected to the C-terminal catalytic region through the switchboard region. Cyclic AMP interacts with the cyclic nucleotide-binding domain (CNBD-B), present in all EPAC isoforms, to trigger enzyme activation. Epac2A has an extra, non-functional cyclic AMP binding domain (CNBD-A). The other functional EPAC domains are indicated; DEP—Dishevelled, Egl-10, Pleckstrin domain, required for protein-protein and protein-lipid interactions; REM—Ras exchange motif, required for the stability of the CDC25-HD catalytic domain; RA—Ras association domain; allows interaction with members of the Ras-superfamily of small GTPases; CDC25-HD—CDC25 homology domain, which contains catalytic GEF activity to Rap 1/2 [14,36,37].