Abstract
Background and objectives
Individuals with ESRD have a very high risk of death. Although mortality rates have decreased over time in ESRD, it is unknown if improvements merely reflect parallel increases in general population survival. We, therefore, examined changes in the excess risk of all-cause mortality—over and above the risk in the general population—among people treated for ESRD in the United States from 1995 to 2013. We hypothesized that the magnitude of change in the excess risk of death would differ by age and RRT modality.
Design, setting, participants, & measurements
We used time-dependent relative survival models including data from persons with incident ESRD as recorded in the US Renal Data System and age-, sex-, race-, and calendar year–specific general population mortality rates from the Centers for Disease Control and Prevention. We calculated relative excess risks (analogous to hazard ratios) to examine the association between advancing calendar time and the primary outcome of all-cause mortality.
Results
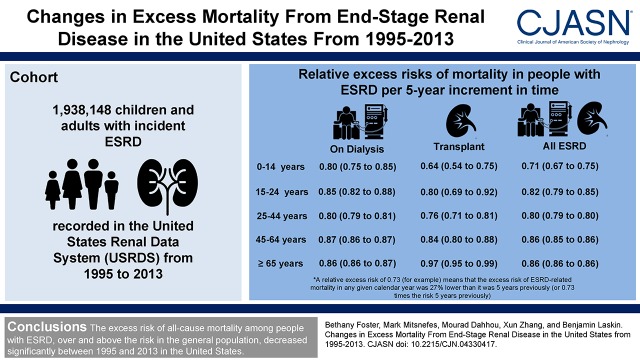
We included 1,938,148 children and adults with incident ESRD from 1995 to 2013. Adjusted relative excess risk per 5-year increment in calendar time ranged from 0.73 (95% confidence interval, 0.69 to 0.77) for 0–14 year olds to 0.88 (95% confidence interval, 0.88 to 0.88) for ≥65 year olds, meaning that the excess risk of ESRD-related death decreased by 12%–27% over any 5-year interval between 1995 and 2013. Decreases in excess mortality over time were observed for all ages and both during treatment with dialysis and during time with a functioning kidney transplant (year by age and year by renal replacement modality interactions were both P<0.001), with the largest relative improvements observed for the youngest persons with a functioning kidney transplant. Absolute decreases in excess ESRD-related mortality were greatest for the oldest persons.
Conclusions
The excess risk of all-cause mortality among people with ESRD, over and above the risk in the general population, decreased significantly between 1995 and 2013 in the United States.
Keywords: Adult; Child; United States; Humans; kidney transplantation; Confidence Intervals; Renal Replacement Therapy; Kidney Failure, Chronic; Risk; kidney; Centers for Disease Control and Prevention (U.S.); renal dialysis
Introduction
ESRD, a condition requiring maintenance dialysis or a kidney transplant for survival, carries a high risk of death. Accordingly, immense efforts aim to extend the life expectancy of persons with ESRD to match that of their healthy peers (1). Analyses of registry data support that survival of those with ESRD has increased over the past 20–30 years (2–4). However, general population survival has also increased due to public health (e.g., smoking prevention) and medical (e.g., cardiovascular) interventions (5,6). Given that no well designed randomized trials in the ESRD population showed a mortality benefit, it remains unknown if the longer life expectancy observed in registries simply reflects improved general population survival (7).
Relative survival models, which yield relative excess risks analogous to hazard ratios, are commonly used in cancer research to estimate changes over time in mortality attributable to specific cancers (8). We applied time-dependent relative survival modeling to examine changes over time in the excess risk of death in persons with ESRD. Excess risk was defined as the mortality risk in the ESRD population minus the expected risk in the age-, sex-, race-, and calendar year–matched general population (9).
Estimating changes in excess ESRD-related mortality risk determines if investments have influenced disease-specific survival and identifies groups requiring new strategies (8). We examined the association between advancing calendar time and the excess risk of mortality among children and adults initiating ESRD care in the United States between 1995 and 2013. We hypothesized that excess mortality decreased over calendar time for all age groups, except adolescents and young adults (due to poor adherence with treatment), and that the magnitude of the decrease differed by RRT modality (dialysis versus transplant).
Materials and Methods
Data Sources and Population
We conducted a retrospective cohort study of children and adults recorded in the US Renal Data System (USRDS) who initiated ESRD care, defined as receiving maintenance dialysis or a kidney transplant, between January 1, 1995 and December 31, 2013; follow-up ended December 31, 2013. The USRDS includes virtually all people diagnosed with ESRD in the United States (details are in Supplemental Material) (1,3,7). We excluded 8780 persons who died on the date of first ESRD care and 123 persons with a recorded age of >100 years old. General population mortality data needed to determine expected mortality risk were obtained from the US Centers for Disease Control and Prevention. The Montreal Children’s Hospital Institutional Review Board approved the study.
Primary Exposure and Outcome Variables
The primary exposure was current calendar year of observation (time-varying, continuous variable). The primary outcome was all-cause mortality. Deaths are captured using the USRDS Death Notification Form and the National Vital Statistics Database (1,3,7).
Relative Survival Models
We used time-dependent relative survival models (9,10) with time-varying covariates to estimate the relative excess risk of ESRD-related mortality associated with advancing calendar year. Relative survival models are Cox models (time to event). Time zero was the date of first ESRD care. Observation was censored at end of observation or third transplant (to simplify modeling), whichever came first. The dataset was dynamic: both age and calendar year of observation were updated as patients were followed through time. To accomplish this, each patient’s observation was split into multiple intervals on the basis of calendar year using the SAS macro Lexis (11,12). Each patient’s expected hazard of death in each time interval was calculated on the basis of the calendar year–specific United States general population mortality rates for individuals of the same sex, race, and age (matched on the basis of the following categories: <1, 1–4, and 5–9 years and 5-year age intervals thereafter) as the patient was in that interval, the calendar year in that interval, and the duration of the interval (13–15). We assumed a constant hazard in each interval. The hazard function at any given time since first ESRD care was modeled as the sum of the expected hazard and the excess hazard due to ESRD (9).
The first set of models did not adjust for RRT modality; these models provide information on the magnitude of the change in excess ESRD-related mortality risk over time, including the effects of all factors that may have contributed to the change. These factors include changes in the relative proportions of observation time in each RRT modality state (i.e., likelihood of having a functioning kidney transplant—determined by access to transplant and allograft survival) and changes over time in mortality risk during treatment with each RRT modality. To determine whether changes over time in excess ESRD-related mortality risk differed by age, the first models included an interaction between current age category (0–14, 15–24, 25–44, 45–64, and ≥65 years old) and calendar year.
A second set of models also included a time-varying RRT modality variable (peritoneal or hemodialysis versus transplant; RRT modality was updated as each patient was followed over time) and the following interaction terms: RRT modality by calendar year, RRT modality by age, and RRT modality by age by calendar year. These models provided separate estimates of the change in excess mortality risk over time for patients of different ages treated with dialysis and patients of different ages treated with transplant, and they provide information on the contribution of changes to dialysis care and transplant care that resulted directly in a change in mortality risk (i.e., without the contributions of changes in access to transplant or allograft survival).
Initial models were unadjusted (included only the primary exposure—calendar year—and the effect modifiers). To account for possible changes in the distributions of patient characteristics over time, all models were subsequently adjusted for sex, race, socioeconomic status quartile (using median household income by zip code within the 2000 US Census data [3]), insurance status at ESRD initiation, and primary disease or number of comorbidities at ESRD initiation (16). Primary disease and baseline comorbidity were highly correlated; all patients with diabetes as their primary kidney disease also had diabetes as a comorbidity. Therefore, we fitted adjusted models including primary disease or comorbidity separately. Missing covariate values were imputed using multiple imputation (17).
The SAS procedure GENMOD with a Poisson error structure was used to estimate the Cox survival model for excess mortality (9,12). Although the models returned relative excess risks associated with a 1-year increment in calendar year, these were scaled up, such that relative excess risks were reported per 5-year increment in calendar year. Analyses were performed using SAS, version 9.4 (SAS Institute) and S-plus Professional, version 6.1 (TIBCO Software). The SAS procedure PROC MI was used to impute the missing values, and PROC MIANALYZE was used to analyze the imputed complete datasets on the basis of five imputations (18). A two-sided P value <0.05 was considered statistically significant. Proportionality of hazards was assessed by fitting models censored at 5, 10, and 15 years; uniformity of results suggested that proportionality was not violated (19).
Crude and Fitted Calendar Year–Specific Excess Mortality Rates
We calculated the crude excess ESRD-related mortality rate (deaths per 1000 person-years) for each age interval in each calendar year as (observed number of deaths minus expected number of deaths within the same age interval and calendar year) times 1000 and divided by person-years of observation within the calendar year (9). The expected number of deaths was determined on the basis of the age, sex, and race distributions of ESRD person-time within each calendar year and the age-, sex-, race-, and calendar year–specific United States general population mortality rates (13–15). Fitted excess mortality rates were calculated for each calendar year from the results of the relative survival models and the crude excess mortality rates for 2005, when the proportion of incident and prevalent patients with ESRD became stable. Supplemental Material provides detailed methods, and explanatory figures are shown in Supplemental Figures 1–3.
Results
Cohort Characteristics
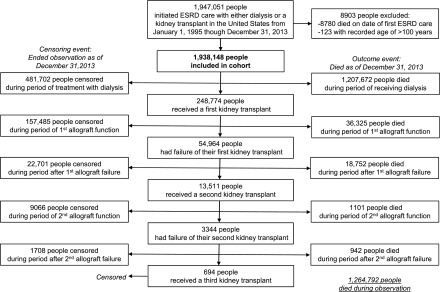
This retrospective cohort study included 1,938,148 children and adults with incident ESRD recorded in the USRDS who initiated care with dialysis or kidney transplant between January 1, 1995 and December 31, 2013; follow-up ended December 31, 2013 (Figure 1). Patients were followed for a median of 2.5 years (interquartile range, 0.8–5.3 years) and could contribute observation to multiple age intervals and calendar years. Table 1 summarizes the composition of the observed experience in consecutive calendar time periods, which reflects, in part, the relative proportions of the total experience in that time period contributed by individuals of different ages. The mean age seemed to decrease slightly over time, as did the proportion of time contributed by people with diabetes and people insured under Medicare/Medicaid. Table 2 summarizes the composition of the observed experience within each age interval as well as the absolute numbers of deaths and crude mortality rates within each age interval. For all age intervals except 0–14 years old, dialysis was the RRT modality for the majority of the observation time; the youngest people spent almost twice as much time with a functioning transplant as on dialysis. Individuals with diabetes and hypertension as their primary disease contributed the largest proportion of observation time in the two oldest age categories, whereas those with GN and congenital anomalies contributed the largest proportion in the youngest ages.
Figure 1.
Flow chart describing the study cohort. The numbers of persons in the cohort who died or were censored at each stage in the trajectory of ESRD care are illustrated.
Table 1.
Composition of the observed experience by calendar period among all children and adults initiating ESRD care from 1995 to 2013 in the United States
| Variable | Calendar Year | |||
|---|---|---|---|---|
| 1995–1999 | 2000–2004 | 2005–2009 | 2010–2013 | |
| Person-years of observation | ||||
| Over all observation | 669,264 | 1,621,412 | 231,9175 | 2,355,051 |
| During treatment with dialysis | 606,392 | 1,340,645 | 1,790,168 | 1,773,237 |
| During time with a functioning transplant | 62,872 | 280,767 | 529,007 | 581,814 |
| Age at first ESRD service, yr | 60 [47–71] | 58 [45–69] | 56 [44–67] | 56 [44–66] |
| Males, % | 54 | 55 | 56 | 57 |
| Race, % | ||||
| White | 61 | 60 | 61 | 61 |
| Black | 32 | 33 | 32 | 31 |
| Other | 7 | 7 | 7 | 8 |
| Primary kidney disease, % | ||||
| Diabetes | 43 | 41 | 40 | 39 |
| Hypertension | 25 | 24 | 24 | 24 |
| GNa | 15 | 16 | 16 | 15 |
| Congenital anomalies of the kidneys and urinary tract | 1 | 2 | 2 | 2 |
| Others | 15 | 16 | 17 | 18 |
| Missing | 1 | 1 | 1 | 1 |
| Socioeconomic quartile, % | ||||
| Lowest | 25 | 24 | 23 | 23 |
| Midlow | 20 | 19 | 19 | 19 |
| Midhigh | 23 | 23 | 24 | 24 |
| Highest | 28 | 29 | 30 | 30 |
| Missing | 4 | 4 | 5 | 5 |
| Primary insurance coverage, % | ||||
| Medicare/Medicaid | 55 | 54 | 51 | 49 |
| Employer/other | 28 | 33 | 35 | 35 |
| No coverage | 8 | 10 | 11 | 11 |
| Missing | 8 | 3 | 3 | 5 |
| Comorbidities,b % | ||||
| None | 34 | 36 | 37 | 38 |
| 1 | 32 | 31 | 31 | 30 |
| 2–3 | 27 | 26 | 25 | 24 |
| ≥4 | 7 | 6 | 6 | 6 |
Because the unit of analysis was person-time rather than person, the characteristics presented are weighted by a factor derived from the number of person-years of observation and number of events and presented as weighted median [interquartile range] or percentage (for example, percentage of person-years contributed by men). Over-representation of the experience during the first year of ESRD care in the 1995–1999 period may influence the estimates for that period.
Includes FSGS.
Comorbidities counted included congestive heart failure, ischemic heart disease/coronary artery disease, myocardial infarction, cardiac arrest, cardiac dysrhythmia, pericarditis, atherosclerotic heart disease, other cardiac disease, cerebrovascular disease/cerebrovascular accident/transient ischemic attack, peripheral vascular disease, diabetes, chronic obstructive pulmonary disease, current tobacco use, malignancy, alcohol dependence, drug dependence, inability to ambulate, and inability to transfer. If no comorbidities were reported, it was assumed that there were none.
Table 2.
Composition of the observed experience by age category among persons initiating ESRD care from 1995 to 2013 in the United States
| Variable | Age Category, yr | ||||
|---|---|---|---|---|---|
| 0–14 | 15–24 | 25–44 | 45–64 | ≥65 | |
| Person-years of observation | |||||
| Overall observation | 55,467 | 153,316 | 1,170,490 | 3,010,635 | 2,574,543 |
| During treatment with dialysis | 19,671 | 82,286 | 780,408 | 2,300,020 | 2,328,056 |
| During time with a functioning transplant | 35,796 | 71,030 | 390,532 | 710,615 | 246,487 |
| Age at first ESRD service, yr | 5 [2–10] | 18 [15–21] | 34 [28–38] | 53 [47–58] | 70 [66–76] |
| Missing, % | 0 | 0 | 0 | 0 | 0 |
| Males, % | 60 | 56 | 58 | 58 | 53 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Race, % | |||||
| White | 72 | 63 | 54 | 57 | 67 |
| Black | 18 | 27 | 38 | 35 | 26 |
| Other | 11 | 10 | 8 | 8 | 7 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Primary kidney disease, % | |||||
| Diabetes | <1 | 2 | 25 | 45 | 45 |
| Hypertension | 1 | 8 | 22 | 22 | 30 |
| GNa | 23 | 43 | 30 | 14 | 9 |
| Congenital anomalies of the kidneys and urinary tract | 39 | 18 | 3 | 1 | <1 |
| Others | 32 | 26 | 18 | 18 | 16 |
| Missing | 5 | 4 | 1 | 1 | 1 |
| Socioeconomic quartile, % | |||||
| Lowest | 18 | 21 | 24 | 25 | 21 |
| Midlow | 16 | 18 | 19 | 19 | 19 |
| Midhigh | 23 | 23 | 24 | 23 | 24 |
| Highest | 33 | 30 | 28 | 28 | 32 |
| Missing | 9 | 8 | 5 | 5 | 4 |
| Primary insurance coverage, % | |||||
| Medicare/Medicaid | 53 | 40 | 35 | 39 | 76 |
| Employer/other | 40 | 42 | 42 | 45 | 16 |
| No coverage | 4 | 14 | 20 | 13 | 3 |
| Missing | 4 | 5 | 4 | 3 | 5 |
| Comorbidities,b % | |||||
| None | 92 | 89 | 60 | 36 | 25 |
| 1 | 6 | 9 | 28 | 33 | 31 |
| 2–3 | 2 | 2 | 11 | 25 | 34 |
| ≥4 | <1 | <1 | 1 | 6 | 10 |
| Deaths, n | |||||
| Overall observation | 1151 | 3365 | 63,123 | 358,485 | 838,668 |
| During treatment with dialysis | 947 | 3048 | 59,601 | 340,804 | 822,966 |
| During treatment with transplant | 204 | 317 | 3522 | 17,681 | 15,702 |
| Observed absolute mortality rate per 1000 person-years (95% CI) | |||||
| Over all observation | 20.8 (12.5 to 29.0) | 21.9 (17.0 to 26.9) | 53.9 (52.2 to 55.7) | 119.1 (118.0 to 120.1) | 325.8 (324.8 to 326.8) |
| During treatment with dialysis | 48.1 (34.5 to 61.8) | 37.0 (30.3 to 43.7) | 76.4 (74.2 to 78.5) | 148.2 (147.0 to 149.4) | 353.5 (352.5 to 354.5) |
| During treatment with transplant | 5.7 (4.6 to 16.0) | 4.5 (2.9 to 11.8) | 9.0 (5.9 to 12.1) | 24.9 (22.6 to 27.2) | 63.7 (59.9 to 67.5) |
Because the unit of analysis was person-time rather than person, the characteristics presented are weighted by a factor derived from the number of person-years of observation and number of events and presented as weighted median [interquartile range] or percentage (for example, percentage of person-years contributed by men). 95% CI, 95% confidence interval.
Includes FSGS.
Comorbidities counted included congestive heart failure, ischemic heart disease/coronary artery disease, myocardial infarction, cardiac arrest, cardiac dysrhythmia, pericarditis, atherosclerotic heart disease, other cardiac disease, cerebrovascular disease/cerebrovascular accident/transient ischemic attack, peripheral vascular disease, diabetes, chronic obstructive pulmonary disease, current tobacco use, malignancy, alcohol dependence, drug dependence, inability to ambulate, and inability to transfer. If no comorbidities were reported, it was assumed that there were none.
The composition of the observed experience in consecutive calendar periods by age category is shown in Supplemental Tables 1–5. These tables provide the best indication of factors that may confound the association between calendar year and excess mortality risk within each age interval. Comorbidity burden seemed stable, but diabetes as the primary cause of ESRD decreased in frequency over time in persons 15–64 years old; the proportion lacking insurance decreased over time in those younger than 24 years old.
Changes in the Excess Risk of ESRD-Related Mortality over Calendar Time
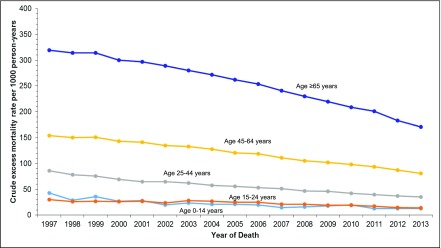
Supplemental Table 6 shows observed and expected numbers of deaths and crude observed and excess mortality rates by age and calendar year. Changes in crude and fitted excess mortality rates over time are illustrated in Figure 2 and Supplemental Figure 4, respectively.
Figure 2.
Crude excess mortality rates decreased over calendar time, for all ages, in people with ESRD. Crude excess ESRD-attributable mortality rates by calendar year are shown within each current age category. Excess mortality rates were calculated by subtracting age-, sex-, race- (white, black, or other), and calendar year–specific United States general population mortality rates (expected) from the observed ESRD mortality rates. Observed mortality rates were compared with general population rates in the following age intervals: <1, 1–4, and 5–9 years and 5-year intervals thereafter. The years 1995 and 1996 are excluded from the plot, because these years include primarily the observation of patients with incident ESRD. Because mortality rates are highest in the initial months after ESRD onset, calendar years in which observation is mainly from incident patients will overestimate excess mortality compared with years in which observation represents a mix of incident and prevalent patients. Fitted excess mortality rates on the basis of the relative excess risks determined by the models and the observed excess mortality rates in 2005, when the proportion of incident and prevalent patients became stable, are shown in Supplemental Figure 4.
Table 3 shows results of unadjusted and adjusted models. In analyses considering all ESRD observation (without adjustment for RRT modality), the excess ESRD-related mortality risk decreased significantly for all age groups. The magnitude of the changes differed significantly by age (year by age interaction P<0.001). Compared with those ≥65 years old, all other age intervals, except 45–64 years old, showed significantly larger relative decreases in excess mortality risk over time. Relative decreases in excess mortality were largest for 0–14 year olds. However, as illustrated in Figure 2, absolute decreases in excess mortality were greatest for the oldest persons.
Table 3.
Relative excess risks of mortality in people with ESRD per 5-year increments in calendar time from 1995 to 2013
| Age, yr | Relative Excess Risk (95% Confidence Interval) | ||
|---|---|---|---|
| Unadjusted | Adjusted, Including Primary Disease | Adjusted, Including Comorbidity | |
| All ESRD observation timea | |||
| 0–14 | 0.75 (0.70 to 0.79) | 0.73 (0.69 to 0.77) | 0.71 (0.67 to 0.75) |
| 15–24 | 0.86 (0.83 to 0.89) | 0.83 (0.80 to 0.86) | 0.82 (0.79 to 0.85) |
| 25–44 | 0.83 (0.82 to 0.84) | 0.82 (0.81 to 0.83) | 0.80 (0.79 to 0.80) |
| 45–64 | 0.89 (0.88 to 0.89) | 0.88 (0.87 to 0.88) | 0.86 (0.85 to 0.86) |
| ≥65 | 0.89 (0.88 to 0.89) | 0.88 (0.88 to 0.88) | 0.86 (0.86 to 0.86) |
| During treatment with dialysisb | |||
| 0–14 | 0.83 (0.78 to 0.89) | 0.81 (0.76 to 0.86) | 0.80 (0.75 to 0.85) |
| 15–24 | 0.89 (0.85 to 0.92) | 0.86 (0.83 to 0.89) | 0.85 (0.82 to 0.88) |
| 25–44 | 0.83 (0.82 to 0.83) | 0.82 (0.81 to 0.83) | 0.80 (0.79 to 0.81) |
| 45–64 | 0.89 (0.88 to 0.89) | 0.88 (0.88 to 0.89) | 0.87 (0.86 to 0.87) |
| ≥65 | 0.88 (0.88 to 0.88) | 0.87 (0.87 to 0.87) | 0.86 (0.86 to 0.87) |
| During treatment with transplantb | |||
| 0–14 | 0.67 (0.56 to 0.79) | 0.65 (0.55 to 0.76) | 0.64 (0.54 to 0.75) |
| 15–24 | 0.85 (0.73 to 0.98) | 0.81 (0.70 to 0.93) | 0.80 (0.69 to 0.92) |
| 25–44 | 0.79 (0.73 to 0.84) | 0.78 (0.74 to 0.83) | 0.76 (0.71 to 0.81) |
| 45–64 | 0.88 (0.83 to 0.92) | 0.86 (0.82 to 0.90) | 0.84 (0.80 to 0.88) |
| ≥65 | 1.00 (0.98 to 1.02) | 0.99 (0.97 to 1.01) | 0.97 (0.95 to 0.99) |
Data are relative excess risks (95% confidence intervals). A relative excess risk of 0.73 (for example) means that the excess risk of ESRD-related mortality in any given calendar year was 27% lower than it was 5 years previously (or 0.73 times the risk 5 years previously). Model fit was assessed using the chi-squared test for goodness of fit, which showed no evidence of significant lack of fit.
The unadjusted model included age and a calendar year by age interaction. Both adjusted models also included sex, race, socioeconomic status quartile, and insurance status at ESRD initiation. The model shown in column 3 also included primary disease; the model shown in column 4 also included number of comorbidities at ESRD initiation.
The unadjusted model included age (time dependent) and RRT modality (time dependent) as well as the following interaction terms: calendar year by age, calendar year by RRT modality, RRT modality by age, and calendar year by age by RRT modality. Both adjusted models also included sex, race, socioeconomic status quartile, and insurance status at ESRD initiation. The model shown in column 3 also included primary disease; the model shown in column 4 also included number of comorbidities at ESRD initiation.
The change in excess mortality risk also differed significantly by RRT modality (both year by RRT modality and year by age by RRT modality interactions P<0.001). Focusing on time during treatment with dialysis, excess mortality risk decreased significantly for all ages, with the greatest relative improvements in the youngest persons (Table 3). During time with a functioning transplant, excess mortality risk decreased for all ages. Differences by age in the relative change in excess mortality risk over time were greater during treatment with transplant than dialysis.
To investigate the possibility that mortality rates for 15–24 year olds did not change until the most recent years (on the basis of Figure 2), we considered additional models in a cohort restricted to those initiating ESRD care from 1995 to 2006, with observation censored at 2006. Between 1995 and 2006, there was no significant change in excess mortality risk among 15–24 year olds considering all ESRD observation (relative excess risk, 0.95; 95% confidence interval, 0.88 to 1.02), during treatment with dialysis (relative excess risk, 1.02; 95% confidence interval, 0.95 to 1.11), or during time with a functioning transplant (relative excess risk, 0.89; 95% confidence interval, 0.63 to 1.27). For all other age groups, changes in excess mortality risk from 1995 to 2006 were similar in magnitude and direction to those seen from 1995 to 2013.
Effects of Covariates
Covariates independently associated with the risk of ESRD-related mortality were age, RRT modality, insurer, socioeconomic status, sex, race, primary disease, and comorbidity (Supplemental Table 7).
Discussion
Among almost 2 million individuals initiating ESRD care in the United States from 1995 to 2013, the excess risk of ESRD-related mortality decreased significantly with advancing calendar time. Improvements were observed across all age groups in analyses considering all ESRD experience. Although it is not possible to determine the exact reasons for the improvements, we speculate that technical advances in dialysis, new medications, and uptake of clinical practice guidelines may have contributed (3,16,20). Increased access to transplantation, especially among the youngest and oldest people with ESRD, and improved allograft survival may also have played a role (1), because mortality rates are substantially lower during treatment with transplant than dialysis (3,16,21).
We found significant differences by age and RRT modality in the magnitude of the changes in excess mortality risk over time. Younger people showed significantly larger relative improvements in excess mortality risk than older people. This was observed for those treated with dialysis, those treated with transplant, and without adjustment for RRT modality. The reasons for greater relative improvements in younger people are not clear. Incomplete capture of comorbidities by the database may have resulted in underestimation of improvements in older patients; comorbidities are more common in older than younger patients, and therefore, inconsistencies in reporting of comorbidities may have a greater effect on results in older patients. Of note, the absolute magnitude of the improvements was largest for the oldest individuals.
Young people with functioning kidney transplants showed greater relative reductions in excess mortality risk over time than young people treated with dialysis. In contrast, elderly people treated with dialysis showed larger relative survival improvements over time than elderly people treated with kidney transplants. The very small or absent improvements among elderly transplant recipients may reflect selection bias. It is possible that only the healthiest elderly people had access to transplants in more remote years, whereas comparatively sicker people were accepted as kidney transplant candidates more recently.
The improvements over time observed during treatment with dialysis or a functioning kidney transplant suggest that advances in clinical care have improved outcomes. No well designed randomized trial in the ESRD population, including trials targeting higher hematocrit values (22), targeting lower intact parathyroid hormone concentrations (23), aiming for a higher dialysis dose, or using high-flux dialyzers (24), showed a survival benefit. However, it may be unrealistic to expect a single intervention to affect mortality risk in a complex condition like ESRD. The cumulative effect of multiple care advances may explain the decreases in excess mortality.
Some hypothesize that improved survival in the ESRD population simply reflects improved survival of the general population (7). Relative survival models remove general population risk from the equation by comparing ESRD-related death rates over time. Two prior studies used a relative survival approach to examine changes in ESRD-specific survival; both concluded that improvements in ESRD-attributable survival did not keep pace with improvements in the general population (25,26). One study compared excess mortality rates in 1987, 1997, and 2007 with rates in 1977, when computer technology was in its infancy; there was significant potential for incomplete death data for 1977, leading to unreliably low mortality estimates (26). The other study focused exclusively on cardiovascular mortality among adults treated with dialysis (25). Neither assessed differences in the change in excess mortality risk by age or RRT modality using time-dependent relative survival models.
In our analyses restricted to the interval between 1995 and 2006, 15–24 year olds were the only age group with no significant improvement in relative survival. This age interval includes a developmental stage associated with poor health outcomes due to decreased adherence to treatment and gaps in care when transferring from pediatric to adult providers (27–29). Increasing awareness of the high risk associated with this age period in the early 2000s (30) and growing implementation of formal transition programs may have contributed to the apparent improvements in survival from 2006 to 2013. Starting in late 2005, the Share 35 policy provided children younger than 18 years old with increased access to kidneys from younger deceased donors, also possibly contributing to the observed improvements (31). It is also conceivable there was insufficient power to detect very small improvements between 1995 and 2006 in this age interval.
To our knowledge, this is the first study to determine the change in excess ESRD-related mortality risk across the entire age range and compare changes over time by age and RRT modality. Another novel feature was the treatment of age, calendar year, and RRT modality as time-dependent variables; this approach allowed us focus on age, calendar year, and RRT modality at death rather than at initiation of ESRD care.
The study does have limitations. Like any retrospective analysis of a registry, our study is subject to limitations due to data entry errors, residual confounding by variables not captured or incompletely captured in the USRDS, and missing data. In particular, comorbidity reporting may be incomplete, resulting in underestimation of the burden of illness in some. Comorbidities are captured by indicating which of a list of comorbidities are present; if none are selected, it is assumed that none are present. It was not possible to determine if an “absent” comorbidity was truly absent or was simply not reported (missing). To minimize the effect of missing data, we used multiple imputation methods, but we cannot exclude the possibility that missingness influenced results. We can also not exclude the possibility that completeness of comorbidity reporting changed over time. Furthermore, changes in comorbidity over time in individual patients are not captured—only comorbidity at ESRD care initiation.
The USRDS may not capture all patients with ESRD, specifically those who died early in the course of dialysis, those who withdrew from dialysis before death, and those with advanced CKD who died before starting dialysis. We also acknowledge that factors other than improvements in care may have influenced the changes observed in excess ESRD-related mortality risk. First, it is possible that improvements in general care or public health measures (i.e., smoking reduction) may have had a bigger effect in the ESRD population than in the general population. Second, selection of healthier patients for RRT in more recent years may also have contributed (32,33). In addition, it is possible that the improvements observed among those being treated with dialysis may be partly due to increasing waiting times for transplant in some age categories, resulting in healthier patients on dialysis continuing dialysis longer in more recent than more remote years.
Among people being treated for ESRD in the United States between 1995 and 2013, the risk of death over and above that expected on the basis of age-, sex-, race-, and calendar year–specific general population rates has dropped significantly. A lack of improvement in the risk of death with a functioning transplant among elderly kidney transplant recipients requires further investigation. Although most trials have not identified individual therapies that improve survival, our findings are encouraging, suggesting that cumulative efforts to improve care have resulted in improved survival. However, absolute mortality rates remain high in ESRD, and continued efforts to improve outcomes are needed.
Disclosures
None.
Supplementary Material
Acknowledgments
B.J.F., a member of the McGill University Health Centre Research Institute (supported in part by the Fonds de la recherche du Québec Santé [FRQS]), received salary support from the FRQS. This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. M.M.M. is supported by National Institutes of Health grant K24DK090070, and B.L.L. is supported by National Institutes of Health grant K23DK101600.
The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Life Expectancy Gains for Patients with ESRD,” on pages 11–12.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04330417/-/DCSupplemental.
References
- 1.United States Renal Data System : USRDS 2014 Annual Data Report: Atlas Of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 2.McDonald SP, Craig JC; Australian and New Zealand Paediatric Nephrology Association : Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ: Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990-2010. JAMA 309: 1921–1929, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Ojo AO, Port FK, Arndorfer JA, Cibrik DM, Kaplan B: Survival improvement among patients with end-stage renal disease: Trends over time for transplant recipients and wait-listed patients. J Am Soc Nephrol 12: 1293–1296, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, Neff L: Current cigarette smoking among adults--United States, 2005-2013. MMWR Morb Mortal Wkly Rep 63: 1108–1112, 2014 [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie CD, Wigington C, Hong Y; Centers for Disease Control and Prevention (CDC) : Coronary heart disease and stroke deaths - United States, 2009. MMWR Suppl 62[3]: 157–160, 2013 [PubMed] [Google Scholar]
- 7.Foley RN, Collins AJ: The USRDS: What you need to know about what it can and can’t tell us about ESRD. Clin J Am Soc Nephrol 8: 845–851, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Rutter CM, Johnson EA, Feuer EJ, Knudsen AB, Kuntz KM, Schrag D: Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst 105: 1806–1813, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickman PW, Sloggett A, Hills M, Hakulinen T: Regression models for relative survival. Stat Med 23: 51–64, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Clayton D, Hills M: Time-varying explanatory variables. In: Statistical Methods in Epidemiology, Oxford, United Kingdom, Oxford University Press, 1993, pp 307–318 [Google Scholar]
- 11.Carstensen B: Lexis Macro, 2007. Available at: http://www.bendixcarstensen.com/Lexis/Lexis.sas. Accessed April 7, 2016
- 12.Dickman P: Estimating and Modelling Relative Survival Using SAS, 2004. Available at: www.pauldickman.com/survival/sas/relative_survival_using_sas.pdf. Accessed April 7, 2016
- 13.Centers for Disease Control and Prevention/National Center for Health Statistics: Death Rates for 113 Selected Causes by 5-Year Age Groups, Race, and Sex: United States, 1979-98. Age Groups <1 Year to 40-44 Years, National Vital Statistics System, HIST002R_1, 2014. Available at: http://www.cdc.gov/nchs/nvss/mortality/hist290.htm. Accessed December 23, 2014
- 14.Centers for Disease Control and Prevention/National Center for Health Statistics: Death Rates for 113 Selected Causes by 5-Year Age Groups, Race, and Sex: United States, 1979-98. Age Groups 45-49 Years to 85+ Years, National Vital Statistics System, HIST002R_2, 2014. Available at: http://www.cdc.gov/nchs/nvss/mortality/hist290.htm. Accessed December 23, 2014
- 15.Centers for Disease Control and Prevention/National Center for Health Statistics: Underlying Cause of Death 1999-2013 on CDC WONDER Online Database, Released 2014. Data Are from the Multiple Cause of Death Files, 1999-2013, as Compiled from Data Provided by the 57 Vital Statistics Jurisdictions through the Vital Statistics Cooperative Program, 2014. Available at: http://wonder.cdc.gov/ucd-icd10.html. Accessed April 1, 2015
- 16.Laskin BL, Mitsnefes MM, Dahhou M, Zhang X, Foster BJ: The mortality risk with graft function has decreased among children receiving a first kidney transplant in the United States. Kidney Int 87: 575–583, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafer JL: Multiple imputation: A primer. Stat Methods Med Res 8: 3–15, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Graham JW, Olchowski AE, Gilreath TD: How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci 8: 206–213, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Allison PD: Survival Analysis Using SAS: A Practical Guide, Cary, NC, SAS Institute, 1995 [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group : KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9[Suppl 3]: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Foster BJ, Dahhou M, Zhang X, Platt RW, Hanley JA: Change in mortality risk over time in young kidney transplant recipients. Am J Transplant 11: 2432–2442, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS; EVOLVE Trial Investigators : Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367: 2482–2494, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R; Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Roberts MA, Polkinghorne KR, McDonald SP, Ierino FL: Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis 58: 64–72, 2011 [DOI] [PubMed] [Google Scholar]
- 26.van Walraven C, Manuel DG, Knoll G: Survival trends in ESRD patients compared with the general population in the United States. Am J Kidney Dis 63: 491–499, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Samuel SM, Nettel-Aguirre A, Soo A, Hemmelgarn B, Tonelli M, Foster B: Avoidable hospitalizations in youth with kidney failure after transfer to or with only adult care. Pediatrics 133: e993–e1000, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Foster BJ, Dahhou M, Zhang X, Dharnidharka VR, Conway J, Ng VL: High risk of liver allograft failure during late adolescence and young adulthood. Transplantation 100: 577–584, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Foster BJ: Heightened graft failure risk during emerging adulthood and transition to adult care. Pediatr Nephrol 30: 567–576, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Watson AR: Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol 14: 469–472, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Amaral S, Patzer RE, Kutner N, McClellan W: Racial disparities in access to pediatric kidney transplantation since share 35. J Am Soc Nephrol 23: 1069–1077, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Ellwood AD, Jassal SV, Suri RS, Clark WF, Na Y, Moist LM: Early dialysis initiation and rates and timing of withdrawal from dialysis in Canada. Clin J Am Soc Nephrol 8: 265–270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosansky SJ, Clark WF: Has the yearly increase in the renal replacement therapy population ended? J Am Soc Nephrol 24: 1367–1370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.