Abstract
Spermatozoa retrieved from the testis of men with high levels of sperm DNA fragmentation (SDF) in the neat semen tend to have better DNA quality. Given the negative impact of SDF on the outcomes of Assisted Reproductive Technology (ART), an increased interest has emerged about the use of testicular sperm for intracytoplasmic sperm injection (Testi-ICSI). In this article, we used a SWOT (strengths, weaknesses, opportunities, and threats) analysis to summarize the advantages and drawbacks of this intervention. The rationale of Testi-ICSI is bypass posttesticular DNA fragmentation caused by oxidative stress during sperm transit through the epididymis. Hence, oocyte fertilization by genomically intact testicular spermatozoa may be optimized, thus increasing the chances of creating a normal embryonic genome and the likelihood of achieving a live birth, as recently demonstrated in men with high SDF. However, there is still limited evidence as regards the clinical efficacy of Testi-ICSI, thus creating opportunities for further confirmatory clinical research as well as investigation of Testi-ICSI in clinical scenarios other than high SDF. Furthermore, Testi-ICSI can be compared to other laboratory preparation methods for deselecting sperm with damaged DNA. At present, the available literature supports the use of testicular sperm when performing ICSI in infertile couples whose male partners have posttesticular SDF. Due to inherent risks of sperm retrieval, Testi-ICSI should be offered when less invasive treatments for alleviating DNA damage have failed. A call for continuous monitoring is nonetheless required concerning the health of generated offspring and the potential complications of sperm retrieval.
Keywords: intracytoplasmic sperm injection, male infertility, sperm DNA fragmentation, sperm retrieval, SWOT analysis, testicular sperm
INTRODUCTION
Male infertility is usually associated with the presence of abnormal semen parameters.1,2 However, sperm dysfunctions other than those revealed by conventional semen analysis may be responsible for the difficulty to conceive.3,4 Sperm DNA plays a critical role in normal embryo development as the genetic information passed on to the next generation depends on sperm DNA integrity.5,6 Among DNA lesions, two main types are of utmost clinical importance, namely single-strand (SS-DB) and double-strand DNA breaks (DS-DB).6
Sperm DNA fragmentation (SDF), a broad term encompassing both SS-DB and DS-DB, is more common in infertile patients than in fertile counterparts.7 Several etiological factors have been implicated in the impairment of sperm DNA content, including environmental and lifestyle factors, varicocele, male accessory gland infections, advanced paternal age, and systemic diseases.8,9,10,11 Furthermore, recent evidence indicates that elevated SDF is associated with poor assisted reproductive outcomes.12 Although sperm with fragmented DNA may fertilize an egg with apparently similar efficiency as sperm without DNA fragmentation, the negative impact of a damaged paternal chromatin to the integrity of embryonic genome is usually observed after implantation.13 This type of damage is often manifested by early pregnancy loss.11 It has been speculated that SDF might also lead to a higher risk of congenital disabilities in the offspring.14,15
The most commonly used tests to measure DNA fragmentation in human sperm are terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), sperm chromatin dispersion test (SCD), single cell gel electrophoresis (Comet) assay, and sperm chromatin structure assay (SCSA). These methods measure the proportion of sperm with either real DNA breaks (e.g., TUNEL) or a combination of real DNA breaks and potentially denaturable DNA due to the preexistence of SS-DB or DS-DB (e.g., SCSA, SCD, and Comet). Probes or dyes are used to identify DNA breaks with the aid of fluorescence microscopy, optical microscopy, and flow cytometry according to the method type. These assays, however, are not interchangeable as they measure different aspects of SDF - though they are interrelated to a greater or lesser extent through properties of the DNA. Comprehensive reviews as regard the characteristics of SDF testing can be found elsewhere.10,16,17
SDF testing has been proposed as complementary to the information provided by routine semen analysis.6,12,18,19 Moreover, the values of SDF can be clinically informative for Assisted Reproductive Technology (ART) outcomes, as discussed above and recently acknowledged by the Practice Committee of the American Society for Reproductive Medicine.2 It is therefore suggested that SDF testing reflected the quality of the entire semen specimen, not just the damaged sperm detected in the test result.
In the presence of abnormal values, several strategies have been proposed to overcome SDF in couples undergoing ART. Varicocele repair,20 oral antioxidant therapy,21 short ejaculatory abstinence periods22 and recurrent ejaculations,23 and laboratory sperm selection techniques such as magnetic cell sorting (MACS),24 physiological intracytoplasmic sperm injection (PICSI),25 and intracytoplasmic morphologically selected sperm injection (IMSI)26 have been attempted. Although some interventions might be potentially useful, including removal of highly apoptotic sperm by MACS and alleviation of oxidative status by oral antioxidants in men with low antioxidant levels, none of them, alone or combined, have been unequivocally proven to fully bypass the potential detrimental effect of abnormal SDF on ART outcomes.2 Notwithstanding, the methods employed to reduce SDF are usually applied to an unselected population of men undergoing ART regardless of SDF rates. A strict inclusion criterion, for instance, of only men with high SDF and grouped by etiology might have avoided diluting the effect size of some of these techniques, a hypothesis that deserves further investigation.
Recently, the use of testicular rather than ejaculated sperm for intracytoplasmic sperm injection (ICSI) among men with high SDF has gained increased attention due to reports of better pregnancy outcomes.27,28 In this review, we summarize the studies that have been published since the first report of the use of testicular sperm for ICSI (Testi-ICSI) among men with high SDF. Moreover, we aim to explore the biological plausibility as regards the potential advantages of Testi-ICSI and try to offer suggestions for its safe use and directions for future research. For this, we applied the SWOT (strengths, weaknesses, opportunities, and threats) analysis, a method usually used in business but also adaptable to reproductive medicine.29 The SWOT analysis framework helps focus on the strengths, understand the weaknesses that can assist in managing and minimizing the threats, and take the greatest possible advantage of the opportunities available (Figure 1).
Figure 1.
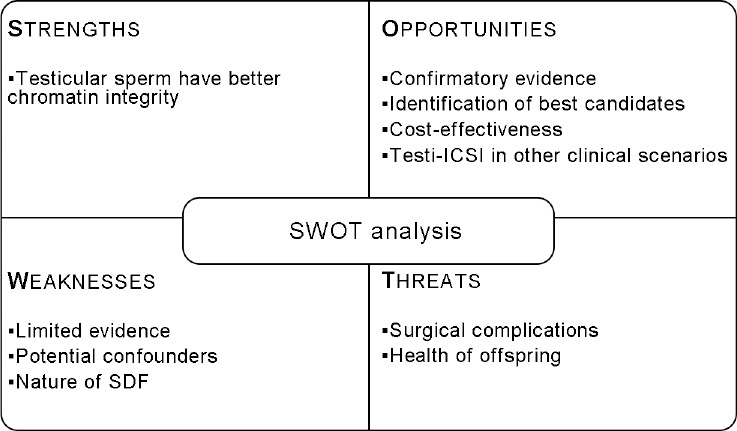
A strength-weakness-opportunities-threats analysis of using testicular sperm for ICSI in infertile men with high sperm DNA fragmentation.
STRENGTHS
Testicular sperm has better chromatin integrity than ejaculated sperm
Moskovtsev et al.30 evaluated DNA damage in ejaculated and testicular spermatozoa of 12 men with persistently high DNA damage despite taking oral antioxidants for 3 months. They compared the values of DNA fragmentation assessed by TUNEL between testicular sperm obtained by testicular sperm extraction (TESE) and ejaculated sperm collected from the same patients on the same day. The rates of SDF in ejaculated sperm were 3-fold higher than testicular sperm (39.7% ± 14.8% vs 13.3% ± 7.3%, P < 0.001). Using the Comet assay, Steele et al.31 showed that the percentage of sperm with intact chromatin was higher (83.0% ± 1.2%) in testicular specimens of twenty men with obstructive azoospermia than in proximal epididymal counterparts (75.4% ± 2.3%; P < 0.05). Along the same lines but using an experimental mice model, Suganuma et al.32 observed that the passage of sperm through the epididymis was associated with a loss of sperm DNA integrity and fertilizing capacity. The characteristics of the studies mentioned above are summarized in Table 1.
Table 1.
Characteristics of studies examining the relationship between sperm DNA fragmentation rates in ejaculated, epididymal, and testicular sperm
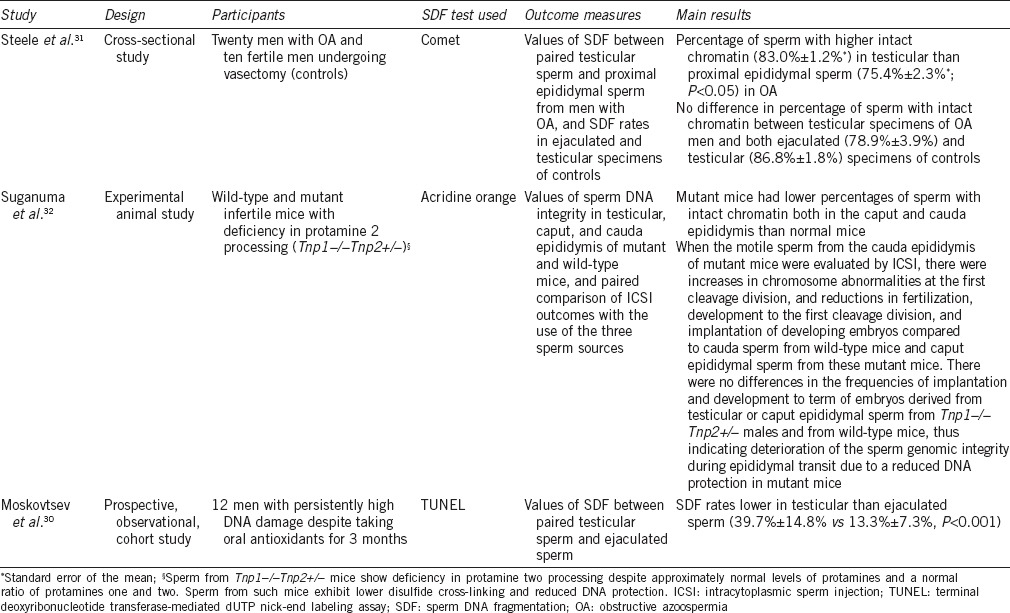
These findings indicated that the main pathways leading to SDF are triggered either during sperm transport through the seminiferous tubules or during the epididymis transit, or both.33 The plausibility of this biological phenomenon relies on three important facts. First, chromatin compaction is still ongoing during epididymal transit. Second, excessive ROS can be generated in the epithelial cells of epididymis under physicochemical stressors such as high temperature and environmental conditions.34,35,36 Finally, some endonucleases can cleave DNA of mature live sperm.37 As a result, sperm DNA damage may ensue through different pathways, including hydroxyl radical, nitric oxide, and activation of sperm caspases and endonucleases, thus explaining the high positivity for SDF in live ejaculated sperm.33 This oxidative-induced damage to the sperm chromatin can be potentially avoided in ICSI candidates provided the epididymis is bypassed. Hence, the use of testicular sperm harvested by testicular sperm aspiration (TESA) or extraction (TESE) becomes attractive as the probability of selecting spermatozoa free of DNA damage for ICSI can be increased. Likewise, the fertilization of an oocyte by a genomically intact testicular spermatozoon will improve the chances of creating a normal embryonic genome that will ultimately increase the likelihood of pregnancy and live birth.27
Testi-ICSI has been associated with improved assisted reproductive technology outcomes
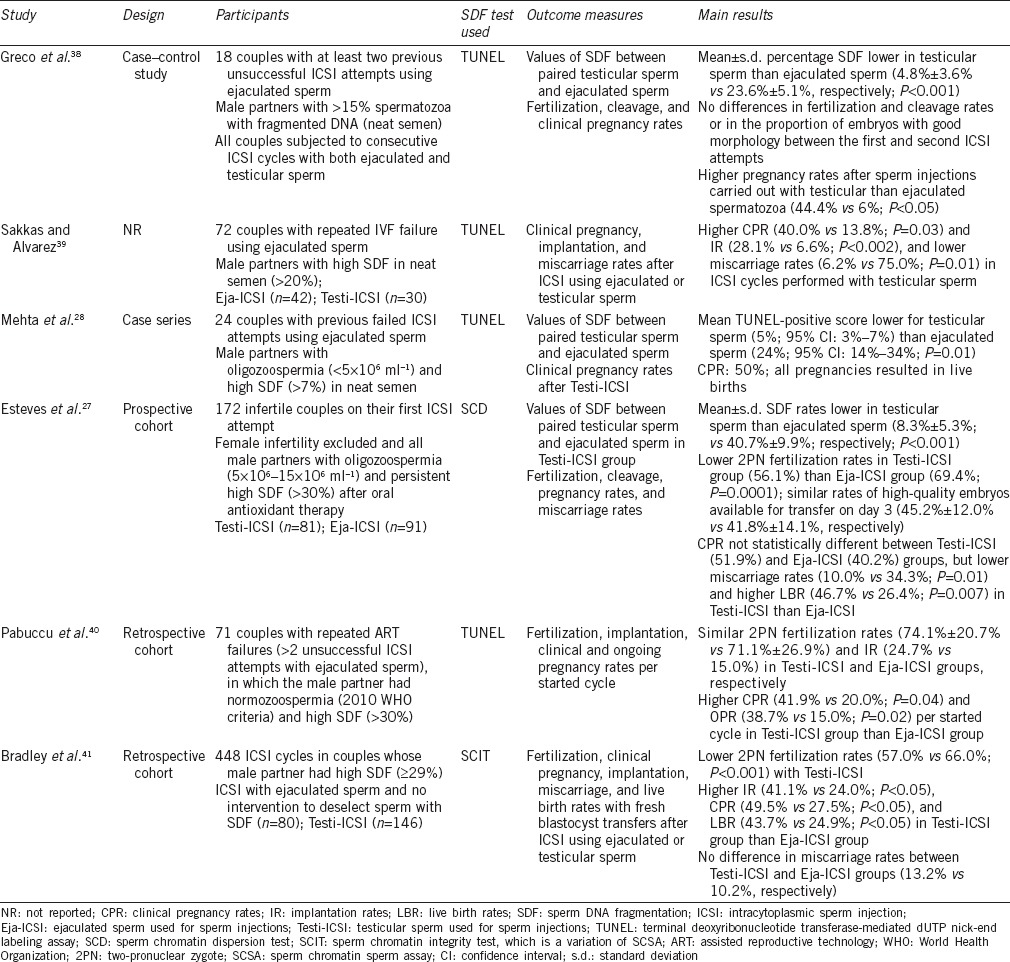
The first report concerning the use of testicular rather than ejaculated sperm for ICSI was published in 2005.38 The authors evaluated 18 couples who had at least two previous unsuccessful ICSI cycles with ejaculated sperm and whose semen evaluation showed >15% spermatozoa with fragmented DNA assessed by TUNEL. Testicular sperm was obtained by testis biopsy, and SDF was evaluated on prepared smears in a similar manner as ejaculated sperm smears. However, in the second ICSI attempt, all sperm injections were performed with testicular sperm. The mean ± s.d. SDF rates in testicular sperm and ejaculated sperm were 4.8% ± 3.6% and 23.6% ± 5.1%, respectively (P < 0.001). There were no significant differences in fertilization and cleavage rates or in the proportion of embryos with good morphology when the first and second ICSI attempts were compared. However, whereas only one pregnancy that spontaneously aborted was obtained in the cycles with ejaculated sperm, eight clinical pregnancies (four singletons and four twins) have been achieved in the cycles carried out with testicular sperm. No miscarriages were recorded after Testi-ICSI.
In 2010, Sakkas and Alvarez showed that pregnancy outcomes were improved using testicular sperm rather than ejaculated sperm in patients with high levels of SDF.39 These authors studied 72 patients with >20% SDF by TUNEL and found higher implantation (P = 0.0021) and clinical pregnancy rates (P = 0.035) and lower miscarriage rates in ICSI cycles performed with testicular sperm. Recently, Mehta et al.28 reported a case series of 24 men with oligozoospermia (<5 × 106 ml−1) and SDF >7% by TUNEL with previous failed ICSI attempts with the use of ejaculated sperm. The patients were subjected to microdissection testicular sperm extraction (micro-TESE) and the retrieved sperm were used for ICSI. Clinical pregnancy was achieved in 50% of 24 couples in the Testi-ICSI cycle and all pregnancies resulted in deliveries of healthy babies. The mean TUNEL-positive level was 24.5% for ejaculated sperm and 4.6% for testicular sperm. In another recent study, Pabuccu et al.40 retrospectively analyzed the outcomes of ICSI from 71 couples with repeated ART failures (>2 unsuccessful ICSI attempts), in which the male partner had normozoospermia (2010 WHO criteria) and high SDF (>30%) by TUNEL. Testicular and ejaculated sperm were used for ICSI in 31 and 40 couples, respectively. Despite similar fertilization and implantation rates, the clinical (41.9% vs 20.0%; P = 0.04) and ongoing (38.7% vs 15.0%; P = 0.02) pregnancy rates per started cycle were higher when testicular sperm were used in preference over ejaculated sperm.
In the only prospective comparative study published to date, Esteves et al.27 compared treatment outcomes between ejaculated and testicular sperm by evaluating a cohort of 172 infertile men with elevated SDF subjected to ICSI. In this study, the authors enrolled infertile men with mild-to-moderate idiopathic oligozoospermia (5 × 106–15 × 106 spermatozoa per ml) presenting with persistent high SDF (>30%) despite oral antioxidant therapy with a combination of Vitamins C, E, folic acid, selenium, and zinc for 3 months. On the day of oocyte retrieval, SDF was assessed in all patients after 2–3 days of ejaculatory abstinence using the sperm chromatin dispersion (SCD) test. In the group of patients undergoing sperm retrieval, which was carried out by either TESE or TESA, SDF was also assessed in testicular specimens using the SCD method combining a dual fluorescent probe to target both the DNA and proteins. This approach allowed for discrimination between spermatozoa and other cell elements in testicular suspensions. The rates of SDF in the group of men subjected to sperm retrieval were 5-fold higher in the semen (40.7% ± 9.9%) than in the testis (8.3% ± 5.3%; P < 0.001); in this group, all sperm injections were performed with testicular sperm. On the contrary, SDF rates were 40.9% ± 10.2% in the group of patients subjected to ICSI with ejaculated sperm. The comparison groups were similar concerning male and female demographic characteristics. However, the miscarriage rates were lower whereas the live birth rates were higher in the couples subjected to sperm injections with testicular sperm. The adjusted relative risk for miscarriage and live birth between testicular and ejaculated groups was 0.29 (95% CI: 0.10–0.82; P = 0.019) and 1.76 (95% CI: 1.15–2.70; P = 0.008), respectively. To our knowledge, this is the study published to date comparing SDF and ICSI outcomes in couples whose male partner had elevated SDF.
The characteristics of the studies discussed above are summarized in Table 2.
Table 2.
Characteristics of studies examining the outcomes of ICSI after the use of testicular and ejaculated sperm among men with high SDF
WEAKNESSES
Limited evidence
The available evidence favoring the use of testicular sperm for ICSI in cases with high SDF is still limited. Most studies have evaluated a small cohort of men or lacked a control group.28,38,39 Moreover, the validity of comparing the results of repeat ICSI cycles with previous unsuccessful ones is scientifically questionable. Only one prospective comparative study, albeit not randomized, was powered to detect differences in live birth rates.27 Notwithstanding, the available evidence may offer the opportunity to develop a potentially interesting systematic review and meta-analysis to compare ICSI outcomes for ejaculated versus testicular sperm among men with high sperm DNA fragmentation in semen.
Potential confounders
The predictive value of SDF for ART success is confounded by several factors. Foremost among these is perhaps the issue of the oocyte quality, which is related to maternal age, and its capability of repairing DNA damage before the first cleavage.42 Furthermore, sperm DNA quality deteriorates with advanced paternal age.43 These factors may further exacerbate the deleterious effect of SDF in ART cycles performed in women of advanced age and in those with reduced ovarian reserve.44 Importantly, these observations indicate that female factor infertility represents an relevant confounder to be controlled by strict inclusion criteria, subgroup analysis, or statistical methods.
Nature of SDF
Testicular sperm may not always overcome the problem of SDF. It is well known that sperm DNA damage can occur in the seminiferous tubules as a result of apoptosis or due to defects in chromatin remodeling during spermiogenesis.6 Intratesticular apoptosis induced by an impairment in sperm maturation leads to early DNA damage; these spermatozoa go through the genital tract without being further damaged by oxidative stress.33 Consequently, the advantage of testicular sperm over ejaculated sperm as regards decreasing SDF is likely to be restricted to posttesticular SDF.
OPPORTUNITIES
Confirmatory evidence
SDF has been shown to contribute to infertility in up to 30% of men.7,45 Among couples undergoing ICSI, abnormally high levels of SDF are found in approximately 32% of the cases.14 And despite being usually associated with abnormal semen parameters, a significant proportion of men (20%–40%) with otherwise normal semen parameters have high SDF.46,47 Therefore, a large proportion of infertile men to be enrolled in ART can potentially benefit from Testi-ICSI.
Still, the evidence concerning the advantage of using testicular over ejaculated sperm to overcome infertility in ICSI candidates with high seminal SDF values is limited, as already discussed. Hence, a call for confirmatory evidence using a prospective approach is needed. For instance, if the aim is to confirm a difference of 10% in the primary outcome measure (e.g., live birth rates), assuming this difference as clinically significant, a minimum of 770 patients (385 per group) will be required to have an 80% chance of detecting, as significant at the 5% level, an increase in live birth rates (i.e., from 30% live birth rates in the control group to 40% in the experimental group). The use of SDF testing and Testi-ICSI will represent an important clinical contribution to doctors and patients alike if the increased likelihood of success is confirmed in such trial, thus reassuring the validity of Testi-ICSI.
Identification of best candidates for Testi-ICSI
Posttesticular damage during sperm transport through the epididymis plays a major role in SDF.39 Infertile men with varicocele, for instance, often have higher SDF than their counterparts without varicocele. In such men, reactive oxygen and nitrogen species are released not only in endothelial cells of the dilated pampiniform plexus and testicular cells (developing germ cells, Leydig cells, macrophages, and peritubular cells), but also in the principal cells of the epididymis.48,49 Apart from varicocele, the epididymis can be the origin of SDF in (1) infectious and inflammatory states that may contribute to chronic epididymal dysfunctions and (2) spermatogenesis defects associated with residual cytoplasm and defective protamination, or both. The former can be observed in spinal cord injury,50 postvasectomy reversal,51 and clinical or subclinical epididymitis.52 In these cases, SDF may result from excessive ROS production by spermatozoa themselves in response to a more prolonged epididymal transit or infiltrating polymorphonuclear leukocytes, or both. The latter can be genetically determined or idiopathic and SDF results from the higher susceptibility of DNA to posttesticular degradation by endonucleases.53 Besides, oxidative-induced SDF can also occur postejaculation. In fact, a strong association exists between the presence of male accessory gland infections (MAGI) and seminal ROS levels, and between smoking and excessive seminal plasma leukocytes and ROS; both conditions have been associated with high SDF.54,55 Due to the lack of knowledge about the usefulness of Testi-ICSI in the particular clinical scenarios mentioned above, opportunities exist for investigating the outcomes of Testi-ICSI in subgroups of men more prone to posttesticular sperm DNA damage.
Cost-effectiveness
Given the widespread availability of both SDF testing and urologists performing sperm retrieval, Testi-ICSI could be implemented in the majority of ART units unlike expensive technologies such as IMSI and MACS. In this regard, the cost-effectiveness of Testi-ICSI could be compared to other laboratory preparation methods to deselect sperm with damaged DNA. To our knowledge, there is only one study that compared interventions to deselect sperm with DNA damage. In this report, Bradley et al.41 retrospectively evaluated 448 ICSI cycles from couples whose male partners had high levels of SDF. Sperm injections were performed with either ejaculated sperm or testicular sperm. In the ejaculated sperm group, the authors applied interventions to deselect sperm with DNA fragmentation, namely, IMSI and PICSI, and compared outcomes with a control group of “no intervention.” They also compared the outcomes of ICSI using ejaculated sperm with and without intervention to Testi-ICSI and found higher live birth rates (P < 0.05) with Testi-ICSI (49.8%) than IMSI (28.7%) and PICSI (38.3%). The lowest live birth rates (24.2%) were achieved when no intervention was carried out to deselect sperm with DNA fragmentation (P = 0.020) (Table 2). Unfortunately, this study neither provides data on the reduction of SDF rates by each intervention modality nor evaluates the cost per delivery by each intervention investigated.
Yet, the study of Esteves et al.27 has shown that the number needed to treat (NNT) by testicular compared to ejaculated sperm to obtain an additional live birth per fresh transfer cycles was 4.9 (95% CI: 2.8–16.8). In other words, if we need to treat about five patients with Testi-ICSI to obtain an additional pregnancy per transfer, it means we could avoid one out of five oocyte pick-ups. Obviously, this simplistic estimation does not consider the additional contribution of frozen embryos regarding cumulative pregnancy rates. Apart from the medical benefit, it is equally important to evaluate the economical advantage of a given intervention. Along the same lines, a predictive model could be developed taking into consideration the differences in specific costs per procedures that may differ between clinics and countries. As such, Testi-ICSI seems to be an attractive method to overcome infertility associated with high SDF in need of an in-depth cost-effectiveness analysis.
Cryptozoospermia
Apart from SDF, the use of testicular sperm for ICSI in other scenarios has been poorly studied. To date, only four reports accounting for <300 cycles assessed ICSI outcomes between ejaculated and testicular sperm in cryptozoospermic men.56,57,58,59 Ben-Ami et al.56 reported higher implantation rate (20.7% vs 5.7%; P = 0.003), higher pregnancy rate (42.5% vs 15.1%; P = 0.004), and higher delivery rate (27.5% vs 9.4%; P = 0.028) with testicular sperm in a small cohort of 17 cryptozoospermic men who had undergone multiple unsuccessful ICSI cycles with ejaculated sperm. In another study, Hauser et al.57 studied 13 couples whose male partner had virtual azoospermia or cryptozoospermia subjected to multiple ICSI cycles with ejaculated and fresh and frozen testicular sperm. In this study, fertilization rates (50.0% vs 38.2%, P < 0.05), high-quality embryo rate (65.3% vs 53.2%, P < 0.05), and implantation rates (18.1% vs 5.1%; P = 0.04) favored fresh testicular sperm compared with ejaculated sperm. Along the same lines, Bendikson et al.58 reported a trend toward higher clinical pregnancy rates for testicular sperm in 16 couples undergoing ICSI with ejaculated and testicular sperms. On the contrary, Amirjannati et al.59 showed no differences in fertilization rates and embryo quality between couples undergoing ICSI with ejaculated (208 cycles) and testicular sperms (16 cycles), but pregnancy rates were not taken into account. Recently, the data from these aforementioned studies were summarized in a meta-analysis, which concluded that there were no differences in ICSI pregnancy rates (relative risk [RR]: 0.53, 95% CI: 0.19–1.42, I2 = 67%) or fertilization rates (RR: 0.91, 95% CI: 0.78–1.06, I2 = 73%) between testicular and ejaculated sperm groups.60 The authors concluded that use of testicular sperm rather than ejaculated sperm for ICSI in men with cryptozoospermia is not recommended. However, the included studies have many limitations. Apart from being underpowered to detect clinically significant differences, only one of them has considered live birth rates as the primary outcome. Therefore, the verdict of this meta-analysis should be taken with caution until further sufficiently sized and properly designed studies are developed.
Repeated implantation failure and recurrent pregnancy loss
The published data concerning Testi-ICSI in repeated implantation failure are merely anecdotal. A single report described success with testicular sperm in four couples with multiple ICSI failures.61 As far as recurrent pregnancy loss (RPL) is concerned, the plausibility of Testi-ICSI relies on the positive association between high SDF and miscarriage in IVF/ICSI cycles. In a meta-analysis evaluating 2969 couples, the risk of miscarriage was increased by 2.2 fold when semen specimens with an abnormally high proportion of DNA damage were used for ICSI (95% CI: 1.54–3.03; P < 0.00001).15 In another meta-analysis pooling data from 14 studies, elevated SDF was associated with higher miscarriage rates in ICSI cycles (OR: 2.68; 95% CI: 1.40–5.14; P = 0.003).62 However, none of the studies included in the meta-analyses mentioned above have specifically investigated patients with RPL.
On the contrary, a recent report examined SDF rates by TUNEL among male partners of 112 couples experiencing RPL and control groups of infertile men with abnormal semen parameters and fertile men with normal semen parameters according to the WHO criteria.63 Despite normal semen analysis, SDF was higher in the RPL group compared with fertile controls (18.8% ± 7.0% vs 12.8% ± 5.3%, P < 0.001) and similar to those of infertile patients (20.8% ± 8.9%). The authors also reported a significant positive correlation between the number of RPL events and an elevated level of SDF. Despite this, to our knowledge, there are no published studies specifically investigating the role of testicular sperm in ART patients with RPL.
THREATS
Surgical complications
Sperm retrievals require surgical interventions that are usually carried out on an outpatient basis. The recovery period is 24–72 h, and risks are low but include infection (<1%), hematoma (<5%), and testicular atrophy.64 The potential for intratesticular bleeding after testicular sperm retrieval seems to be higher with open than percutaneous biopsy techniques.65 The more problematic adverse effect of sperm retrieval, namely, reduction in testosterone production an, is caused by the removal of large amounts of testis tissue containing hormone-producing Leydig cells with open surgical techniques (TESE). This effect has been reported in a few men with nonobstructive azoospermia subjected to multiple biopsies.66 However, from a holistic viewpoint, less invasive treatments for men (i.e., ICSI with ejaculated sperm) might represent more invasive treatments for women (i.e., repeat oocyte retrievals) if fewer pregnancies and/or more miscarriages are obtained with ejaculated sperm among men with high SDF.
Health of offspring
While defective spermatozoa passing the testicular barrier can be deselected through natural apoptotic-like process,33 it is possible that defective testicular sperm originating from a subpopulation that would be blocked in its ontogeny during the maturation process is selected for ICSI. Aneuploidy rates were higher in testicular sperm obtained from men with nonobstructive azoospermia than epididymal sperm and ejaculated sperm.67,68 Whereas testicular spermatozoa have overall low DNA damage, this potential advantage could be offset by the higher aneuploidy rates. In one study, Moskovtsev et al.69 compared aneuploidy rates at the testicular and posttesticular levels in the same patients with high SDF. Although SDF rates were almost 3-fold lower in testicular sperm (40.6% ± 14.8% vs 14.9% ± 5.0%, P < 0.05), higher aneuploidy rates for chromosomes 18, 21, X, and Y were observed in testicular spermatozoa than ejaculated sperm (12.41% ± 3.7% vs 5.77% ± 1.2%, P < 0.05).69 Still, the proportion of testicular sperm with aneuploidy was relatively low, and these findings are yet to be confirmed in larger series comprising both men with oligozoospermia and unexplained infertility. Notwithstanding, it might be argued that ICSI candidates represent a particular category of patients that would be unlikely to attain natural reproduction. Therefore, a small increase in the risk of having health issues in the offspring could be acceptable in return of a confirmed beneficial effect of Testi-ICSI, provided the actual number of affected individuals is extremely low. Since the evidence favoring ICSI outcomes with the use of testicular sperm in men with high SDF is limited, a call for continuous monitoring is warranted until the safety of this strategy is confirmed further. In fact, any genetic and epigenetic effects in the offspring will require a more extensive investigation and long-term follow-up.
CONCLUSIONS
Fair evidence indicates that sperm DNA fragmentation is associated with poorer ART outcomes. There is a rationale for the use of testicular sperm for ICSI owing to the improvement in live birth rates in men with persistent high SDF in semen. The biological plausibility of this favorable effect relates to the fact that posttesticular exposure of spermatozoa to oxidative DNA damage in the epididymis is avoided. However, the evidence favoring Testi-ICSI in such cases is still limited as there are no randomized controlled trials comparing ejaculated and testicular sperm. And notably, the current literature does not support the use of testicular in preference over ejaculated sperm for ICSI in clinical scenarios other than high SDF, including extremely low sperm numbers. Despite the potential risks associated with sperm retrieval, ample opportunities exist to confirm the efficacy of Testi-ICSI and further investigate the role of testicular sperm for ICSI in different subgroups of infertile men with and without high DNA damage. Furthermore, the cost-effectiveness of Testi-ICSI as regards the reduction in SDF can be compared with other laboratory methods of sperm selection. At present, the method should be reserved for selected men who have failed less invasive treatments for known and unknown causes of sperm DNA damage, particularly when posttesticular sperm DNA damage is suspected.
REVIEW CRITERIA
We searched PubMed until December 2016 to identify studies evaluating ICSI outcomes among men with high levels of SDF, irrespective of test method and cutoff values utilized, who underwent either consecutive ICSI cycles using both ejaculated sperm and surgically extracted testicular sperm or separate ICSI cycles using ejaculated sperm or testicular sperm. Our search was based on the following key words, alone or combined: “intracytoplasmic sperm injection,” “sperm DNA fragmentation,” “sperm DNA damage,” “sperm chromatin integrity OR damage,” “oligozoospermia,” “normozoospermia,” “testicular OR testicular sperm,” “ejaculated OR ejaculated sperm,” with the filters: “humans,” “English language,” and “Full text.” We excluded studies involving men with a diagnosis of azoospermia and those in which SDF analysis had not been performed. Our search identified five studies, which are summarized in Table 1.
AUTHOR CONTRIBUTIONS
SCE and MR designed the study, participated in the acquisition of data, and drafted the manuscript. NG revised the manuscript and helped in data interpretation and coordination. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014;40:443–53. doi: 10.1590/S1677-5538.IBJU.2014.04.02. [DOI] [PubMed] [Google Scholar]
- 2.Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103:18–25. doi: 10.1016/j.fertnstert.2014.12.103. [DOI] [PubMed] [Google Scholar]
- 3.Hamada A, Esteves SC, Nizza M, Agarwal A. Unexplained male infertility: diagnosis and management. Int Braz J Urol. 2012;38:576–94. doi: 10.1590/s1677-55382012000500002. [DOI] [PubMed] [Google Scholar]
- 4.Esteves SC. A clinical appraisal of the genetic basis in unexplained male infertility. J Hum Reprod Sci. 2013;6:176–82. doi: 10.4103/0974-1208.121419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis SE, Aitken JR, Conner SJ, Iuliis GD, Evenson DP, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online. 2013;27:325–37. doi: 10.1016/j.rbmo.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Gosálvez J, Lopez-Fernandez C, Fernandez JL, Esteves SC, Johnston SD. Unpacking the mysteries of sperm DNA fragmentation: ten frequently asked questions. J Reprod Biotechnol Fertil. 2015;4:1–16. [Google Scholar]
- 7.Spano M, Bonde JP, Hjollund HI, Kolstad HA, Cordelli E, et al. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril. 2000;73:43–50. doi: 10.1016/s0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 8.Feijo CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril. 2014;101:58–63. doi: 10.1016/j.fertnstert.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Esteves SC, Gosálvez J, López-Fernández C, Núñez-Calonge R, Caballero P, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol. 2015;47:1471–7. doi: 10.1007/s11255-015-1053-6. [DOI] [PubMed] [Google Scholar]
- 10.Majzoub A, Esteves SC, Gosálvez J, Agarwal A. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J Androl. 2016;18:205–12. doi: 10.4103/1008-682X.172642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122:497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Cho CL, Esteves SC. Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol. 2016;28:164–71. doi: 10.1097/GCO.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 13.Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004;19:611–5. doi: 10.1093/humrep/deh127. [DOI] [PubMed] [Google Scholar]
- 14.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–9. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 15.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 16.Esteves SC, Sharma RK, Gosálvez J, Agarwal A. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol. 2014;46:1037–52. doi: 10.1007/s11255-014-0715-0. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016;5:935–50. doi: 10.21037/tau.2016.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteves SC. Novel concepts in male factor infertility: clinical and laboratory perspectives. J Assist Reprod Genet. 2016;33:1319–35. doi: 10.1007/s10815-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis SE. Should sperm DNA fragmentation testing be included in the male infertility work-up? Reprod Biomed Online. 2015;31:134–7. doi: 10.1016/j.rbmo.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang YJ, Zhang RQ, Lin YJ, Zhang RG, Zhang WL. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012;25:307–14. doi: 10.1016/j.rbmo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Zini A, San Gabriel M, Baazeem A. Antioxidants and sperm DNA damage: a clinical perspective. J Assist Reprod Genet. 2009;26:427–32. doi: 10.1007/s10815-009-9343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology. 2016;94:102–10. doi: 10.1016/j.urology.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 23.Gosálvez J, González-Martínez M, López-Fernández C, Fernández JL, Sánchez-Martín P. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil Steril. 2011;96:1083–6. doi: 10.1016/j.fertnstert.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Said TM, Grunewald S, Paasch U, Rasch M, Agarwal A, et al. Advantage of combining magnetic cell separation with sperm preparation techniques. Reprod Biomed Online. 2005;10:740–6. doi: 10.1016/s1472-6483(10)61118-2. [DOI] [PubMed] [Google Scholar]
- 25.Parmegiani L, Cognigni GE, Bernardi S, Troilo E, Taraborrelli S, et al. Comparison of two ready-to-use systems designed for sperm-hyaluronic acid binding selection before intracytoplasmic sperm injection: PICSI vs. sperm slow: a prospective, randomized trial. Fertil Steril. 2012;98:632–7. doi: 10.1016/j.fertnstert.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 26.Antinori M, Licata E, Dani G, Cerusico F, Versaci C, et al. Intracytoplasmic morphologically selected sperm injection: a prospective randomized trial. Reprod Biomed Online. 2008;16:835–41. doi: 10.1016/s1472-6483(10)60150-2. [DOI] [PubMed] [Google Scholar]
- 27.Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104:1398–405. doi: 10.1016/j.fertnstert.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Mehta A, Bolyakov A, Schlegel PN, Paduch DA. Higher pregnancy rates using testicular sperm in men with severe oligospermia. Fertil Steril. 2015;104:1382–7. doi: 10.1016/j.fertnstert.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Engmann L, Benadiva C, Humaidan P. GnRH agonist trigger for the induction of oocyte maturation in GnRH antagonist IVF cycles: a SWOT analysis. Reprod Biomed Online. 2016;32:274–85. doi: 10.1016/j.rbmo.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Moskovtsev SI, Jarvi K, Mullen JB, Cadesky KI, Hannam T, et al. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril. 2010;93:1142–6. doi: 10.1016/j.fertnstert.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Steele EK, McClure N, Maxwell RJ, Lewis SE. A comparison of DNA damage in testicular and proximal epididymal spermatozoa in obstructive azoospermia. Mol Hum Reprod. 1999;5:831–5. doi: 10.1093/molehr/5.9.831. [DOI] [PubMed] [Google Scholar]
- 32.Suganuma R, Yanagimachi R, Meistrich ML. Decline in fertility of mouse sperm with abnormal chromatin during epididymal passage as revealed by ICSI. Hum Reprod. 2005;20:3101–8. doi: 10.1093/humrep/dei169. [DOI] [PubMed] [Google Scholar]
- 33.Muratori M, Tamburrino L, Marchiani S, Cambi M, Olivito B, et al. Investigation on the origin of sperm DNA fragmentation: role of apoptosis, immaturity and oxidative stress. Mol Med. 2015;21:109–22. doi: 10.2119/molmed.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 35.Banks S, King SA, Irvine DS, Saunders PT. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction. 2005;129:505–14. doi: 10.1530/rep.1.00531. [DOI] [PubMed] [Google Scholar]
- 36.Rubes J, Selevan SG, Sram RJ, Evenson DP, Perreault SD. GSTM1 genotype influences the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat Res. 2007;625:20–8. doi: 10.1016/j.mrfmmm.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Sotolongo B, Huang TT, Isenberger E, Ward WS. An endogenous nuclease in hamster, mouse, and human spermatozoa cleaves DNA into loop-sized fragments. J Androl. 2005;26:272–80. doi: 10.1002/j.1939-4640.2005.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 38.Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20:226–30. doi: 10.1093/humrep/deh590. [DOI] [PubMed] [Google Scholar]
- 39.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–36. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 40.Pabuccu EG, Caglar GS, Tangal S, Haliloglu AH, Pabuccu R. Testicular versus ejaculated spermatozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous ART failures. Andrologia. 2017 doi: 10.1111/and.12609. Doi: 10.1111/and.12609. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Bradley CK, McArthur SJ, Gee AJ, Weiss KA, Schmidt U, et al. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology. 2016;4:903–10. doi: 10.1111/andr.12215. [DOI] [PubMed] [Google Scholar]
- 42.Meseguer M, Santiso R, Garrido N, García-Herrero S, Remohí J, et al. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95:124–8. doi: 10.1016/j.fertnstert.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 43.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103:9601–6. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji J, Pan C, Fei Q, Ni W, Yang X, et al. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserve. Fertil Steril. 2015;103:910–6. doi: 10.1016/j.fertnstert.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Erenpreiss J, Elzanaty S, Giwercman A. Sperm DNA damage in men from infertile couples. Asian J Androl. 2008;10:786–90. doi: 10.1111/j.1745-7262.2008.00417.x. [DOI] [PubMed] [Google Scholar]
- 46.Saleh RA, Agarwal A, Nelson DR, Nada EA, El-Tonsy MH, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril. 2002;78:313–8. doi: 10.1016/s0015-0282(02)03219-3. [DOI] [PubMed] [Google Scholar]
- 47.Giwercman A, Lindstedt L, Larsson M, Bungum M, Spano M, et al. Sperm chromatin structure assay as an independent predictor of fertility in vivo: a case-control study. Int J Androl. 2010;33:e221–7. doi: 10.1111/j.1365-2605.2009.00995.x. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9:678–90. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 49.Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J Androl. 2016;18:186–93. doi: 10.4103/1008-682X.170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brackett NL, Ibrahim E, Grotas JA, Aballa TC, Lynne CM. Higher sperm DNA damage in semen from men with spinal cord injuries compared with controls. J Androl. 2008;29:93–9. doi: 10.2164/jandrol.107.003574. [DOI] [PubMed] [Google Scholar]
- 51.Smit M, Wissenburg OG, Romijn JC, Dohle GR. Increased sperm DNA fragmentation in patients with vasectomy reversal has no prognostic value for pregnancy rate. J Urol. 2010;183:662–5. doi: 10.1016/j.juro.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Ollero M, Gil-Guzman E, Lopez MC, Sharma RK, Agarwal A, et al. Characterization of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis and treatment of male infertility. Hum Reprod. 2001;16:1912–21. doi: 10.1093/humrep/16.9.1912. [DOI] [PubMed] [Google Scholar]
- 53.Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, et al. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod. 2001;16:1922–30. doi: 10.1093/humrep/16.9.1922. [DOI] [PubMed] [Google Scholar]
- 54.Taha EA, Ez-Aldin AM, Sayed SK, Ghandour NM, Mostafa T. Effect of smoking on sperm vitality, DNA integrity, seminal oxidative stress, zinc in fertile men. Urology. 2012;80:822–5. doi: 10.1016/j.urology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Ochsendorf FR. Infections in the male genital tract and reactive oxygen species. Hum Reprod Update. 1999;5:399–420. doi: 10.1093/humupd/5.5.399. [DOI] [PubMed] [Google Scholar]
- 56.Ben-Ami I, Raziel A, Strassburger D, Komarovsky D, Ron-El R, et al. Intracytoplasmic sperm injection outcome of ejaculated versus extracted testicular spermatozoa in cryptozoospermic men. Fertil Steril. 2013;99:1867–71. doi: 10.1016/j.fertnstert.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 57.Hauser R, Bibi G, Yogev L, Carmon A, Azem F, et al. Virtual azoospermia and cryptozoospermia-fresh/frozen testicular or ejaculate sperm for better IVF outcome? J Androl. 2011;32:484–90. doi: 10.2164/jandrol.110.011353. [DOI] [PubMed] [Google Scholar]
- 58.Bendikson KA, Neri QV, Takeuchi T, Toschi M, Schlegel PN, et al. The outcome of intracytoplasmic sperm injection using occasional spermatozoa in the ejaculate of men with spermatogenic failure. J Urol. 2008;180:1060–4. doi: 10.1016/j.juro.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 59.Amirjannati N, Heidari-Vala H, Akhondi MA, Hosseini Jadda SH, Kamali K, et al. Comparison of intracytoplasmic sperm injection outcomes between spermatozoa retrieved from testicular biopsy and from ejaculation in cryptozoospermic men. Andrologia. 2012;44(Suppl 1):704–9. doi: 10.1111/j.1439-0272.2011.01253.x. [DOI] [PubMed] [Google Scholar]
- 60.Abhyankar N, Kathrins M, Niederberger C. Use of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with cryptozoospermia: a meta-analysis. Fertil Steril. 2016;105:1469–75. doi: 10.1016/j.fertnstert.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Weissman A, Horowitz E, Ravhon A, Nahum H, Golan A, et al. Pregnancies and live births following ICSI with testicular spermatozoa after repeated implantation failure using ejaculated spermatozoa. Reprod Biomed Online. 2008;17:605–9. doi: 10.1016/s1472-6483(10)60306-9. [DOI] [PubMed] [Google Scholar]
- 62.Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnant and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2014;102:998–1005. doi: 10.1016/j.fertnstert.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 63.Carlini T, Paoli D, Pelloni M, Faja F, Dal Lago A, et al. Sperm DNA fragmentation in Italian couples with recurrent pregnancy loss. Reprod Biomed Online. 2017;34:58–65. doi: 10.1016/j.rbmo.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–83. doi: 10.1590/s1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- 65.Esteves SC, Lee W, Benjamin DJ, Seol B, Verza S, Jr, et al. Reproductive potential of men with obstructive azoospermia undergoing percutaneous sperm retrieval and intracytoplasmic sperm injection according to the cause of obstruction. J Urol. 2013;189:232–7. doi: 10.1016/j.juro.2012.08.084. [DOI] [PubMed] [Google Scholar]
- 66.Esteves SC. Clinical management of infertile men with nonobstructive azoospermia. Asian J Androl. 2015;17:459–70. doi: 10.4103/1008-682X.148719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernardine L, Gianaroli L, Fortini D, Conte N, Magli C, et al. Frequency of hyper-hypohaploidy and diplody in ejaculate, epididymal and testicular germ cells of infertile patients. Hum Reprod. 2000;15:2165–72. doi: 10.1093/humrep/15.10.2165. [DOI] [PubMed] [Google Scholar]
- 68.Palermo GD, Colombero LT, Hariprashad JJ, Schlegel PN, Rosenwaks Z. Chromosome analysis of epididymal and testicular sperm in azoospermic patients undergoing ICSI. Hum Reprod. 2002;17:570–5. doi: 10.1093/humrep/17.3.570. [DOI] [PubMed] [Google Scholar]
- 69.Moskovtsev SI, Alladin N, Lo KC, Jarvi K, Mullen JB, et al. A comparison of ejaculated and testicular spermatozoa aneuploidy rates in patients with high sperm DNA damage. Syst Biol Reprod Med. 2012;58:142–8. doi: 10.3109/19396368.2012.667504. [DOI] [PubMed] [Google Scholar]


