Dear Editor,
Trichomonas vaginalis (T. vaginalis), a flagellated protozoan parasite emerged as one of the most common nonviral sexually transmitted infections worldwide, often inhabits the vagina, urethra, prostate, and epididymis.1 It has been estimated that there are more than 170 million new cases of T. vaginalis infections per year worldwide. However, current knowledge of T. vaginalis and trichomoniasis is based mainly on studies in female vaginal infections. The prevalence of trichomoniasis in males is far less well characterized than that in females, probably because the infection seems to be asymptomatic in most men and can be resolved after treatment with one dose of metronidazole.1,2,3
Among men, trichomoniasis has been considered as a cause of nongonoccocal urethritis (NGU) and as involvement in the impairment of male fertility.1,3 T. vaginalis is found more often in infertile men than that in fertile individuals and its presence in semen results in significant decreased sperm parameter values, such as motility, normal morphology and viability.4 In vitro studies have also shown that T. vaginalis and its secretory products reduce sperm motility and fertilizing capacity.4,5 Although T. vaginalis has been identified in urethral discharge, urine, semen, and prostatic fluid, its infection may occur in other areas of the urogenital system. In rare cases reported, T. vaginalis infects the epididymis and prostate gland and occasionally, the testis.4,6,7
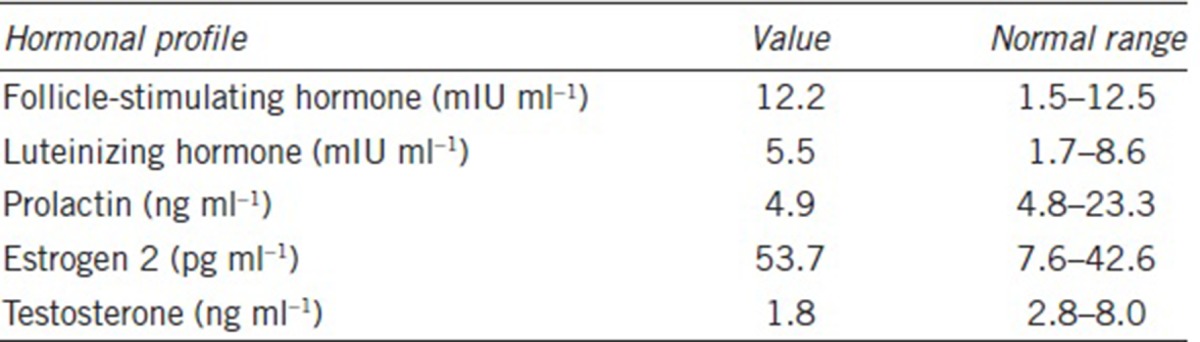
Herein, we report a novel case of nonobstructive azoospermia (NOA) with T. vaginalis infection in the testis. A 32-year-old male patient (1.76 m height and 90 kg weight), married for 10 years, presented with the complaint of infertility. General physical examination was normal and the ultrasound examination demonstrated normal epididymides, vasa deferentia, prostate, seminal vesicles, and ejaculatory duct. The testicular volume of each side was 6 ml, somewhat less than normal. Repeated semen analyses found no spermatozoa in the ejaculate, even after centrifugation. His endocrine profile listed in Table 1 demonstrated that estrogen (E2) was a bit high while testosterone was low, which to a certain extent might be related to his obesity (body mass index [BMI] = 29.05). Genetic analysis showed normal 46, XY karyotype and no microdeletion of Yq azoospermia factor gene. No evidence of gross structural pathology was identified according to the formal urological evaluation and the diagnosis of NOA was given. At the same time, vaginal secretions from his wife were tested to be negative for trichomonas.
Table 1.
Hormonal profile of the patient
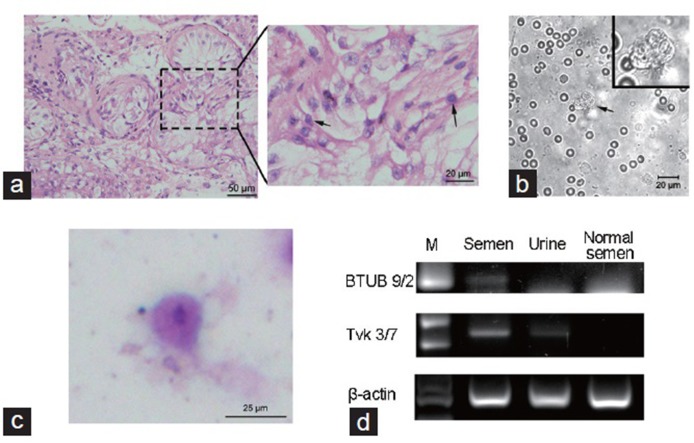
Therefore, we attempted retrieval of the patient's spermatozoa via surgical biopsy of the testes, which would be cryopreserved for further in vitro fertilization (IVF) treatment with his wife's oocytes. Wet preparations from fresh testicular biopsies from four locations in his right testis were examined for sperm under phase contrast microscopy and the pathological examination was performed at the same time. Sections of the testicular biopsies showed that very few germ cells, appearing to be spermatogonia and spermatocytes, were scattered in the seminiferous tubules, while spermatids were rarely detected (Figure 1a). The pathological diagnosis suggested a severe disruption of spermatogenesis. Meanwhile, wet preparations of testicular biopsies failed to demonstrate any sperm cells. However, some flagellated motile protozoa among numerous testicular and red blood cells were observed in one of the wet preparations (Figure 1b and Supplementary Information). In an attempt to identify the protozoa-like structures, Wright-Giemsa staining was made on the same day. On the basis of the morphological features of the cells, namely, an amoeboid shape, the presence of one elliptically shaped nucleus and poorly defined cytoplasm (Figure 1c), a provisional identification of T. vaginalis parasites was made.
Figure 1.
(a) HE staining of testicular biopsies, showed a severe disruption in spermatogenesis. Arrows indicate the germ cells. Scale bars = 50 μm (left) and 20 μm (right). (b) Wet preparation of testicular biopsies, showed a Trichomonas-like flagellate (arrow). Scale bar = 20 μm. (c) Wright-Giemsa staining of the wet preparation smear, arrow indicates Trichomonas vaginalis. Scale bar = 25 μm. (d) PCR analysis of Trichomonas vaginalis from the semen and urine. A normozoospermic semen (normal semen) sample was applied as negative control. M: 100 bp DNA ladder. HE: Hematoxylin-Eosin; PCR: polymerase chain reaction.
Furthermore, laboratory PCR analysis was notable for the identification of this parasite. Briefly, genomic DNA was prepared from 1.5 ml of semen or 5 ml of urine using DNA extract kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer's instructions. PCR was performed to check the expression of Tvk and BTUB, according to a procedure described previously.8,9,10
The results of PCR showed approximately 261 bp fragments representing Tvk 3/7, which has previously been shown to be the most sensitive conventional PCR test for T. vaginalis,8 in the DNA extracts of both the semen and the urine. Likewise, results also showed an approximately 112 bp fragment representing BTUB 9/2, although a bit weak, in the DNA extract of the semen (Figure 1d). Therefore, both semen and urine from the patient were considered positive for T. vaginalis, and the NOA symptom may be caused by T. vaginalis-induced orchitis.
To our knowledge, this clinical case represents the first report of NOA related to T. vaginalis infection at the level of the testis. Combined with the existing reports,3,4,6,7 it illustrates that spermatogenesis failure resulting from T. vaginalis infection in testis may be accompanied by low serum testosterone and atrophic testes, which may reflect the cytotoxicity of T. vaginalis in damaging germ cells and Leydig cells. Therefore, T. vaginalis infection in testis, although occasional, can seriously injure the niche essential for spermatogenesis. Meanwhile, male trichomoniasis is almost asymptomatic and few cases are diagnosed and treated. Hence, the infection persists, and males with a long-term trichomoniasis, such as 8 or 10 years, are more likely to suffer from NOA and become infertile. Although the role of trichomonas infection in pathogenesis of NOA and infertility is still unclear, this case also illustrates the importance of careful diagnosis and timely therapy for trichomoniasis.
On the other hand, it is noteworthy that the rare cases of T. vaginalis infection in the testis may imply a potential defect in the natural defense of the male urogenital tract. As is well known, innate host defense mechanisms in the male genital tract, such as cytokines, epithelial barrier, and epididymal macrophages, are critical for the defense against potential pathogens and provide an appropriate microenvironment for germ cell development and sperm maturation.4 The presence of T. vaginalis in the epididymis, as well as in testis, suggests that the immune barrier is impaired while the protozoa travel through the winding genital tract. Thus, the association between chronic infection by T. vaginalis, inflammation, and a defect in defense of the male reproductive system should receive attention. Above all, it is important to improve the microenvironment of the urogenital tract in defending against pathogens during the therapy for trichomoniasis.
AUTHOR CONTRIBUTIONS
CX and ZL designed the experiment. YHG and YL performed the experimental work and participated in the pathological work. PL, ZJZ, and YH provided assistance in sample collection and treatment. GHF and YJX participated in the pathological work. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study was supported by a key grant from the Joint Research Project for Emerging Frontier (SHDC12015122), Shanghai Shenkang Hospital Applicable Technology Project, the National Key Basic Research Program of China (2015AA020404) and the National Nature Science Foundation of China (81671512).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Mielczarek E, Blaszkowska J. Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection. 2016;44:447–58. doi: 10.1007/s15010-015-0860-0. [DOI] [PubMed] [Google Scholar]
- 2.Sherrard J, Ison C, Moody J, Wainwright E, Wilson J, et al. United Kingdom national guideline on the management of Trichomonas vaginalis 2014. Int J STD AIDS. 2014;25:541–9. doi: 10.1177/0956462414525947. [DOI] [PubMed] [Google Scholar]
- 3.Gimenes F, Souza RP, Bento JC, Teixeira JJ, Maria-Engler SS, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol. 2014;11:672–87. doi: 10.1038/nrurol.2014.285. [DOI] [PubMed] [Google Scholar]
- 4.La Vignera S, Vicari E, Condorelli RA, D’Agata R, Calogero AE. Male accessory gland infection and sperm parameters (review) Int J Androl. 2011;34:e330–47. doi: 10.1111/j.1365-2605.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 5.Roh J, Lim YS, Seo MY, Choi Y, Ryu JS. The secretory products of Trichomonas vaginalis decrease fertilizing capacity of mice sperm in vitro. Asian J Androl. 2015;17:319–23. doi: 10.4103/1008-682X.145070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssenswillen C, Tournaye H, Pierard D, Devroey P, Van Steirteghem A. Microsurgical epididymal sperm aspiration with motile trophozoite cells but no spermatozoa. Hum Reprod. 1997;12:2217–9. doi: 10.1093/humrep/12.10.2217. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd G, Case JR, De Frias D, Brannigan RE. Trichomonas vaginalis orchitis with associated severe oligoasthenoteratospermia and hypogonadism. J Urol. 2003;170:924. doi: 10.1097/01.ju.0000080375.18547.cc. [DOI] [PubMed] [Google Scholar]
- 8.Crucitti T, Van Dyck E, Tehe A, Abdellati S, Vuylsteke B, et al. Comparison of culture and different PCR assays for detection of Trichomonas vaginalis in self-collected vaginal swab specimens. Sex Transm Infect. 2003;79:393–8. doi: 10.1136/sti.79.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pillay A, Lewis J, Ballard RC. Evaluation of Xenostrip-Tv, a rapid diagnostic test for Trichomonas vaginalis infection. J Clin Microbiol. 2004;42:3853–6. doi: 10.1128/JCM.42.8.3853-3856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madico G, Quinn TC, Rompalo A, McKee KT, Jr, Gaydos CA. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J Clin Microbiol. 1998;36:3205–10. doi: 10.1128/jcm.36.11.3205-3210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.