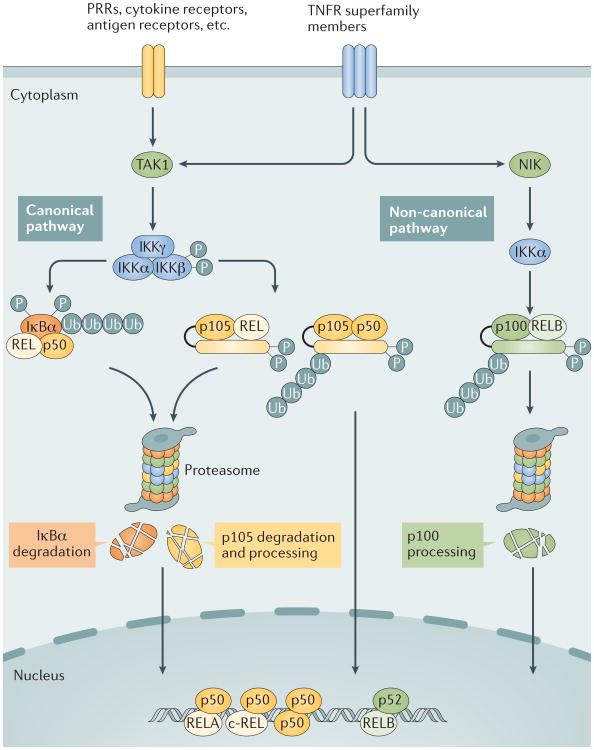
Figure 1. Canonical and non-canonical NF-κB pathways.
The canonical nuclear factor-κB (NF-κB) pathway is triggered by signals from a large variety of immune receptors, which activate the kinase TGFβ -activated kinase 1 (TAK1). TAK1 then activates a trimeric IκB kinase (IKK) complex, composed of catalytic (IKKα and IKKβ) and regulatory (IKKγ) subunits, via phosphorylation of IKKβ. Upon stimulation, the IKK complex, largely through IKKβ, phosphorylates members of the inhibitor of κB (IκB) family, such as the prototypical IκB member IκBα and the I κB-like molecule p105, which sequester NF-κB members in the cytoplasm. IκBα associates with dimers of p50 and members of the REL family (RELA or c-REL), whereas p105 associates with p50 or REL (RELA or c-REL). Upon phosphorylation by IKK, IκBα and p105 are targeted for ubiquitin (Ub)-dependent degradation in the proteasome, resulting in the nuclear translocation of canonical NF-κB family members, which bind to specific DNA elements, termed κB enhancers of target genes, in the form of various dimeric complexes, including RELA–p50, c-REL–p50, and p50–p50 (REF. 1). By contrast, non-canonical NF- κB signalling is based on the processing of p100, an IκB-like molecule that predominantly, although not exclusively, regulates RELB. The non-canonical NF- κB pathway selectively responds to a subset of tumour necrosis factor receptor (TNFR) superfamily members that target the activation of the kinase NFκB-inducing kinase (NIK)4. NIK phosphorylates and activates IKKα, which in turn phosphorylates carboxy-terminal serine residues of p100, triggering selective degradation of the C-terminal IκB-like structure of p100 and leading to the generation of p52 and the nuclear translocation of p52 and RELB4. PRRs, pattern recognition receptors.