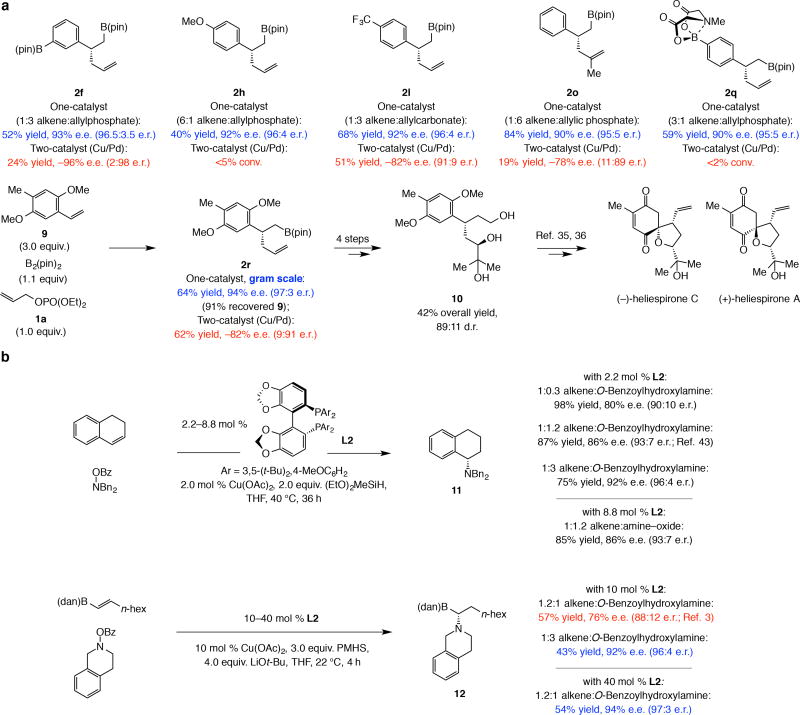
Figure 5. Better mechanistic understanding leads to broader scope.
The mechanistic knowledge obtained through this study allows for a broader scope. a, Without a Pd-based co-catalyst and by adjusting the alkene:electrophile ratio based on the structural attributes of the substrates, the scope of a catalytic process may be significantly broadened. This is illustrated by the representative examples shown here. Experiments were performed at least in triplicate. b, The impact of the findings resulting from the present study extend to transformations involving enantioselective Cu–H addition as the initial step. Thus, higher enantioselectivity, at times significant (e.g., 12 obtained in 92% vs. 76% e.e.) can be achieved by modifying the reaction conditions. Moreover, it is now easier to understand why higher catalyst loading makes little impact in reactions involving an electron rich alkene by have a more substantial effect when an electron-deficient olefin is involved. See the Supplementary Information, Sections 7 and 12, for the complete synthesis route and all experimental and analytical details. pin, pinacolato; Mes, 2,4,6-trimethylphenyl; dppf, 1,1’-bis(diphenylphosphino)ferrocene.
Conditions the same as those in Table 1. Conv. determined by analysis of the 1H NMR spectra of the unpurified mixtures (±2%). Yields are of isolated and purified product (±5%); differences between conv. and yield is due to allyl–B(pin) formation (excess alkene) or of proto-boryl addition products (excess allyl electrophile). Enantioselectivity determined by HPLC analysis (±1%). Experiments were performed at least in triplicate. See the Supplementary Information (Section 5) for details.