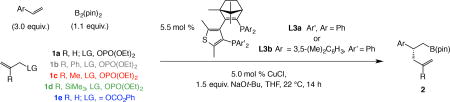
Table 1.
Scope of the catalytic process and the effect of alkene:electrophile ratio.
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Ar; Electrophile | Alkene: Electrophile | Product | Conv. (%)*; Yield (%)† | e.e.§ |
| 1 | Ph; 1a | 3:1 | 2a | >98; 67 | 90 |
| 2 | o-MeOC6H4; 1a | 3:1 | 2b | >98; 55 | 90 |
| 3 | o-FC6H4; 1a | 3:1 | 2c | 98; 54 | 78 |
| 4 | o-F3CC6H4; 1a | 3:1 | 2d | >98; 46 | 66 |
| 5 | 2-naphthyl; 1a | 3:1 | 2e | >98; 65 | 78 |
| 6 | m-(pin)BC6H4; 1a | 3:1 | 2f | >98; 44 | 66 |
| 7 | m-tert-BuO2CC6H4; 1a | 3:1 | 2g | >98; 69 | 64 |
| 8 | p-MeOC6H4; 1a | 3:1 | 2h | >98; 28 | 94 |
| 9 | p-FC6H4; 1a | 3:1 | 2i | >98; 66 | 84 |
| 10 | p-(pin)BC6H4; 1a | 3:1 | 2j | >98; 48 | 64 |
| 11 | p-tert-BuO2CC6H4; 1a | 6:1 | 2k | 22; 14 | 2 |
| 12 | p-F3CC6H4; 1a | 3:1 | 2l | >98; 70 | 16 |
| 13 | 3-Boc-indolyl; 1a | 3:1 | 2m | >98; 58 | 96 |
|
| |||||
| 14 | 2-naphthyl; 1a | 1:3 | 2e | 80; 50 | 92 |
| 15 | m-(pin)BC6H4; 1a | 1:3 | 2f | 71; 52 | 93 |
| 16 | m-tert-BuO2CC6H4; 1a | 1:3 | 2g | 83; 62 | 90 |
| 17 | p-(pin)BC6H4; 1a | 1:3 | 2j | 66; 56 | 84 |
| 18 | p-tert-BuO2CC6H4; 1a | 1:3 | 2k | >98; 72 | 4 |
| 19 | p-F3CC6H4; 1a | 1:3 | 2l | >98; 79 | 34 |
|
| |||||
| 20 | Ph;
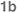
|
3:1 | 2n | >98; 60 | 80 |
| 21 | Ph;
|
1:3 | 2n | >98; 51 | 92 |
| 22 | Ph;

|
3:1 | 2o | 45; 29 | 80 |
| 23 | Ph;

|
1:6 | 2o | >98; 84 | 90 |
| 24 | Ph;

|
3:1 | 2p | >98; 63 | 90 |
| 25 | Ph;

|
1:3 | 2p | 88; 73 | 92 |
|
| |||||
| 26 |
o-FC6H4;

|
1:3 | 2c | 79; 64 | 92 |
| 27 |
o-F3CC6H4;

|
1:3 | 2d | 68; 50 | 92 |
| 28 |
p-FC6H4;

|
3:1 | 2i | >98; 65 | 96 |
| 29 |
p-F3CC6H4;

|
3:1 | 2l | >98; 68 | 92 |
Reactions were carried out under N2 atmosphere with L3a as the chiral ligand, except for L3b in the case of 2n, 2o and 2p).
Conv. determined by analysis of the 1H NMR spectra of the unpurified mixtures (±2%).
Yields are of isolated and purified product (±5%); differences between conv. and yield is due to allyl–B(pin) formation (excess alkene) or of proto-boryl addition products (excess allyl electrophile).
Enantiomeric excess (e.e.) determined by HPLC analysis (±1%). Experiments were performed at least in triplicate. See the Supplementary Information (Section 5) for experimental and analytical details. pin, pinacolato; Boc, tert-butoxycarbonyl.
