Figure 3.
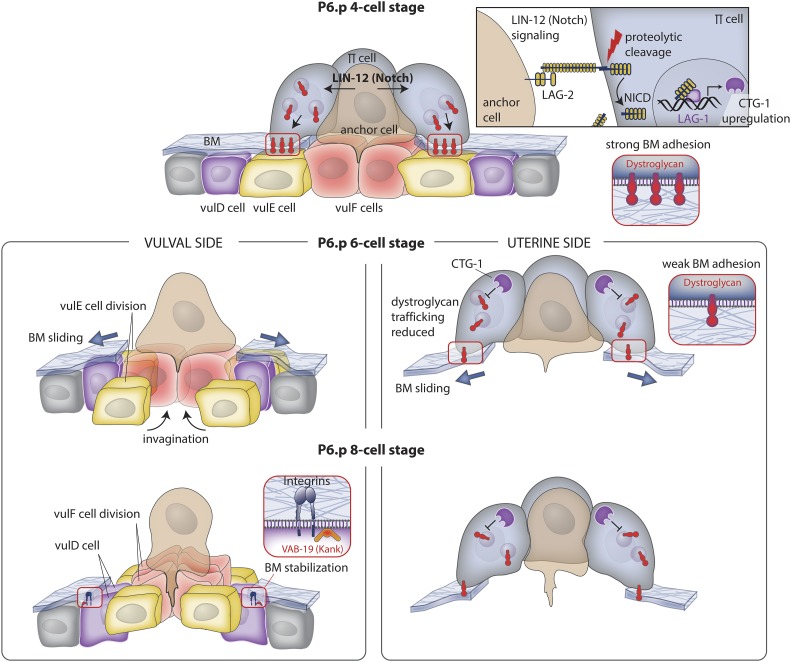
BM sliding following AC invasion widens the breach. Top panel (P6.p 4-cell stage): during invasion, the AC activates LIN-12 (Notch) signaling in neighboring uterine π cells via the ligand LAG-2 (Delta; see upper right box). Notch activation leads to proteolytic release of the LIN-12 (Notch) intracellular domain (NICD), which enters the nucleus, associates with LAG-1 (CSL), and upregulates expression of the gene encoding CTG-1 (Sec14-GOLD protein). Middle panel left (P6.p 6-cell stage): vulval and uterine tissue growth and vulval cell invagination apply forces on the BM that drive its shifting. VulF cells begin to invaginate and the vulE cells divide, lose contact with the BM, and allow the BM to slide over these cells, thus widening the BM gap. Middle panel right: CTG-1 activity in the uterine π cells inhibits the trafficking of the BM adhesion receptor dystroglycan to the cell-BM interface, weakening BM adhesion, and allowing the BM to move on the uterine side of the BM. Lower panel left (P6.p 8-cell stage): the vulF cells divide and further invaginate with the vulE cells. The BM stops shifting over the nondividing vulD cell, which sets the width of the opening of the BM gap. Integrin and VAB-19 (KANK) localize to the vulD-BM interface to stabilize BM adhesion. Lower panel right: dystroglycan levels continue to be reduced at the interface, allowing the BM to slide to a position determined by the underlying vulD cell.