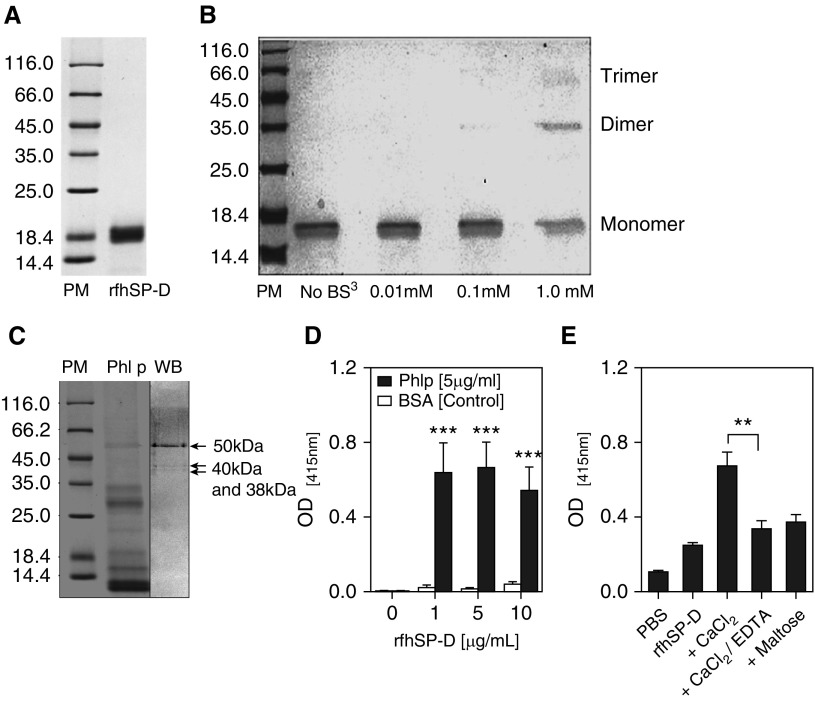
Figure 1.
Purification of recombinant fragment of human surfactant protein D (rfhSP-D) and characterization of its binding to Phleum pratense (Phl P) extract (A) by 15% vol/vol sodium dodecyl sulfate–polyacrylamide gel electrophoresis showing purified rfhSP-D protein at approximately 20 kD. (B) Trimerization of rfhSP-D was achieved at 1 mM concentration of bis(sulfosuccinimidyl) suberate (BS3) cross-linking agent. (C) Far Western blot (WB) showing that rfhSP-D binds to three P. pratense proteins (50 kD, 40 kD, and 38 kD). Lane 1 = protein marker (PM); lane 2 = P. pratense extract (Phl p); lane 3 = Western blot. (D) rfhSP-D binds to P. pratense extract. (E) The binding of rfhSP-D to P. pratense extract is calcium and carbohydrate dependent and is inhibited by 5 mM ethylenediaminetetraacetic acid (EDTA) and 5 mM maltose. Data are presented as median (interquartile range) and are representative of five to seven independent experiments. **P < 0.01; ***P < 0.001. BSA = bovine serum albumin; OD = optical density; PBS = phosphate-buffered saline.