Abstract
Aims/Introduction
The impact of body mass index on mortality among patients with diabetes remains controversial. Therefore, we carried out a meta‐analysis of pertinent studies.
Materials and Methods
We searched OVID/MEDLINE, EMBASE and Cochrane databases for all reported studies, which investigated the relationship between body mass index and mortality in patients with diabetes. Summary estimates of hazard ratios (HRs) were obtained with a random effects model. Univariate meta‐regressions were carried out.
Results
A total of 20 studies including 250,016 patients with diabetes were identified. The results of the present study showed a significantly reduced risk of all‐cause mortality in overweight patients (HR 0.82, 95% CI: 0.74–0.91, P < 0.0001, and I 2 = 91.6%) as compared with normal weight patients. The survival benefits of obesity were only observed in the elderly patients (HR 0.69, 95% CI: 0.63–0.75, P < 0.0001, and I 2 = 50.4%), but not in the younger patients (HR 1.01, 95% CI: 0.84–1.20, P = 0.96, I 2 = 80.1%). Furthermore, the beneficial prognostic impacts on overweight (coefficient = 0.030, P = 0.041) and obesity (coefficient = 0.032, P = 0.010) were attenuated with clinical follow‐up duration.
Conclusions
The present meta‐analysis showed a significantly lower risk of all‐cause mortality in overweight patients with diabetes compared with normal weight patients. However, the survival benefits of obesity were only observed among the elderly patients.
Keywords: Body mass index, Diabetes, Mortality
Introduction
Obesity is a growing healthcare concern worldwide. Epidemiological studies show that >85% of patients with type 2 diabetes are overweight or obese1, 2. The detrimental effects of obesity on metabolism and insulin resistance have been well documented, and obesity is also closely tied to the etiology of type 2 diabetes2, 3. Losing weight has been proven to improve insulin sensitivity and metabolic control for overweight or obese patients, and thus has been recommended in the treatment of diabetes4, 5. Recently, the phenomenon of the ‘obesity paradox,’ which refers to a lower risk of mortality for overweight or obese patients assessed by body mass index (BMI), has been reported in a variety of populations, such as patients with cardiovascular disease6, 7, heart failure8 and chronic kidney disease9. However, the influence of BMI on mortality among patients with diabetes remains controversial. Although several studies have also shown an inverse correlation of weight with mortality among patients with diabetes10, 11, 12, some studies suggest no correlation or a direct correlation13, 14. Heterogeneity of the study population, follow‐up duration, varied obesity measurements and a limited number of events in some studies are all considered to contribute to the controversy among these studies. Among various obesity measurements, BMI assessment is cheap, simple and widely used across the globe. Therefore, in order to thoroughly appraise the relationship of BMI and the mortality for patients with diabetes, we carried out a meta‐analysis of pertinent studies.
Methods
Two investigators (FG, ZJW) independently searched OVID/MEDLINE, EMBASE and the Cochrane library databases (Cochrane Central Register of Controlled Trials) for all reported studies, published before December 2014, with English‐only citations, which investigated the relationship between body mass index (BMI) categories and mortality in patients with diabetes. The search terms and their synonyms related to bodyweight (e.g., ‘obesity,’ ‘overweight,’ ‘body weight,’ ‘body mass index’), diabetes (e.g., ‘diabetes mellitus,’ ‘diabetes,’ ‘diabetics,’ ‘hyperglycemia’) and relevant clinical end‐points (e.g., ‘mortality,’ ‘death,’ ‘survival rate’) were combined with terms related to study design (e.g., ‘cohort study,’ ‘longitudinal study,’ ‘clinical trial’). We also used the Science Citation Index to cross‐reference for studies that met our criteria. Citations were initially screened at the title level, followed by the abstract level and finally were retrieved as full texts. Studies were excluded if they met any one of the following criteria: (i) duplicate publication; (ii) ongoing/unpublished study; (iii) publication only as an abstract or as conference proceedings; or (iv) trials only assessed the BMI as the continuous variable. A flow diagram as to the process of study selection is shown in Figure 1. The study was carried out according to the guidelines of the Meta‐analysis of Observational Studies in Epidemiology Group for the conduct of meta‐analyses of intervention studies15.
Figure 1.
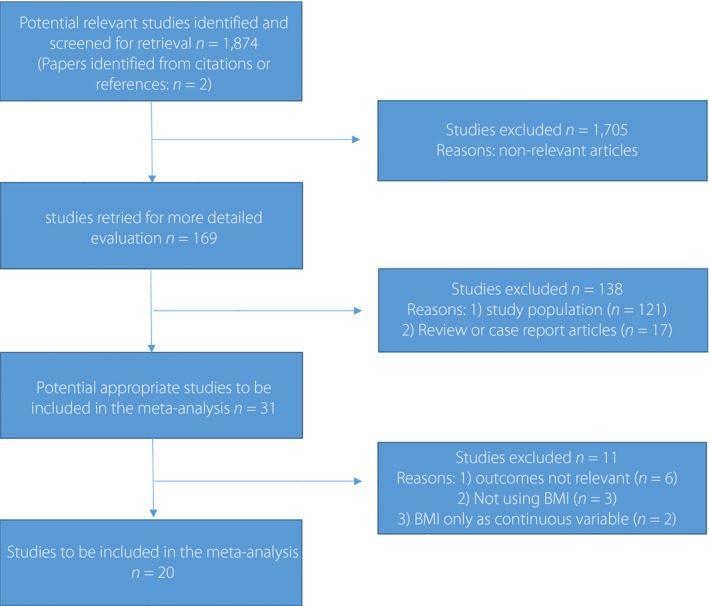
Flow chart of the meta‐analysis. BMI, body mass index.
The classifications of BMI were based on the World Health Organization's criteria16, normal weight as 18.5–25, overweight as 25–30 and obesity as >30. However, the standard categories were not used by some studies. To avoid missing important information, we included the studies in which BMI categories were within 2 kg/m2 of the standard categories. Studies evaluating the risk of mortality for overweight/obese patients vs normal weight or non‐overweight patients were included as well. Separate analyses were carried out to compare the results for studies with or without standard BMI classifications. The Newcastle–Ottawa scale was used to assess the risk of bias in individual studies17. This scale rates studies based on eight criteria. We made a modification by removing the criterion of ‘demonstration that outcome of interest was not present at start of study.’ The primary outcome of the meta‐analysis was all‐cause mortality. The secondary outcome was cardiovascular mortality.
Statistical analysis
Individual study hazard ratios (HRs) and 95% confidence intervals (95% CI) were extracted for each article. Summary estimates of HRs were obtained with a random effects model if significant heterogeneity was found (I 2 > 50%). The heterogeneity across the trials was calculated with the I 2 statistic18. Sensitivity analyses were examined by excluding one study at a time. Univariate meta‐regressions were carried out, and variables included the total number of patients, total number of mortality events, mean age, incident or prevalent diabetes, standard or non‐standard BMI classifications and follow‐up period. Publication bias was explored by visual inspection of a funnel plot and the Begg and Mazumdar's rank correlation test19. Analyses were carried out using the Comprehensive Meta Analysis Version 2.0 (Biostat, Englewood, NJ, USA). The statistical level of significance for the summary treatment effect estimate was a two‐tailed P‐value <0.05.
Results
From 1,874 potentially relevant citations, 20 studies10, 11, 12, 13, 14, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, including 250,016 patients with diabetes, met the criteria for the analysis. Quality assessment of each included study is listed in Table S1. The follow‐up duration of these studies ranged from 2.9 to 16.7 years. The baseline characteristics of the studies are shown in Table 1.
Table 1.
Characteristics of included studies
| Study | Publication time | Location | Population | Incident or prevalent diabetes | No. patients | Follow‐up duration (years) | Age, mean (years) | BMI category (kg/m2) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Thomas et al.12 | 2014 | UK |
Type 2 diabetes Two cohorts: with prior CVD; without prior CVD |
Incident | 47,509 (37,272 + 10,237) | 5 |
60 (without prior CVD) 67 (with prior CVD) |
18.5–24.9; 25.0–29.9; ≥30 |
All‐cause mortality |
| Lajous et al.20 | 2014 | France | Women with type 2 diabetes | Incident | 2,421 | 16.7 | – |
<25 ≥25 |
All‐cause mortality |
| Bozorgmanesh et al.14 | 2014 | Iran | Type 2 diabetes | Incident | 1,322 | 9.1 | 53.6 | Tertiles (median) 24.9 28.9 33.8 | All‐cause mortality |
| Jackson et al.33 | 2014 | US | Diabetes | Prevalent | 4,740 | 9 | 50.1 | 22.84–25.09; 25.10–27.46; 27.47–31.02; 31.03–54.92 | All‐cause mortality |
| Murphy et al.11 | 2014 | Iceland | Type 2 diabetes | Prevalent | 637 | 6.7 | 76 | 18.5–24.9; 25.0–29.9; ≥30 | All‐cause mortality |
| Tobias et al.13 | 2014 | USA | Type 2 diabetes | Incident | 11,427 | 15.8 | 62 | 18.5–22.4; 22.5–24.9; 25.0–27.4; 27.5–29.9; 30.0–34.9; ≥35.0 | All‐cause mortality |
| Waring et al.20 | 2011 | USA | Type 2 diabetes | Prevalent | 1,644 | 5 | 74 |
18.5–24.9; 25–29.9; 30–34.9; 35–39.9; ≥40 |
All‐cause mortality |
| Perotto et al.21 | 2013 | Italy |
Type 2 diabetes Two cohorts: <65 years ≥65 years |
Prevalent | 1,475 | 15 | – |
<24.2; 24.3–26.7; 26.8–30.0; >30.0 |
All‐cause mortality, Cardiovascular mortality |
| Eeg‐Olofsson et al.22 | 2009 | Sweden |
Type 2 diabetes With no prior CVD or stroke |
Prevalent | 13,087 | 5.6 | 60.3 |
<25; 25–29.9; ≥30; |
All‐cause mortality |
| Weiss et al.23 | 2009 | Israel | Diabetes | Prevalent | 121 | 3.7 | 79 |
<22.1; 22.11–24.95; 24.96–27.5; ≥27.51 |
All‐cause mortality |
| Tseng24 | 2013 | Taiwan |
Type 2 diabetes Two cohorts: female; male |
Prevalent | 89,056 | 12 |
61.4 (female) 59.7 (male) |
18.5–22.9; 23.0–24.9; 25.0–29.9; ≥30 |
All‐cause mortality |
| Carnethon et al.10 | 2012 | USA | Type 2 diabetes | Incident | 2,625 | – | 41–76 | 18.5–24.9; ≥25.0 | All‐cause mortality |
| Sluik et al.25 | 2010 | Europe | Diabetes | Prevalent | 5,435 | 9.3 |
57.4 (male) 57.6 (female) |
≤24.9; 25.0–27.1; 27.2–29.1; 29.2–31.8; ≥31.9 |
All‐cause mortality Cardiovascular mortality |
| Mulnier et al.26 | 2006 | UK | Type 2 diabetes | Prevalent | 44,230 | 7 | 65.8 |
20–24; 25–29; 30–34; 35–54 |
All‐cause mortality |
| Church et al.27 | 2004 | USA | Diabetes | Prevalent | 2,196 | 14.6 | 49.3 |
<25.0; 25.0–29.9; ≥30.0 |
All cause mortality |
| Doehner et al.28 | 2012 | Europe | Type 2 diabetes | Prevalent | 5,202 | 2.9 | 62 |
22–25; 25–30; 30–35; ≥35 |
All‐cause mortality |
| Kokkinos et al.29 | 2012 | USA | Type 2 diabetes | Prevalent | 4,156 | 7.5 | 60 | 18.5–24.9; 25.0–29.9; 30.0–34.9; >35 | All‐cause mortality |
| Ford et al.30 | 1991 | USA | Diabetes | Prevalent | 602 | 10 | – |
<27.8; 27.8–31.1; ≥31.1 (male) <27.3; 27.3–32.3; ≥32.3 (female) |
All‐cause mortality Cardiovascular mortality |
| McEwen et al.31 | 2007 | USA | Diabetes | Prevalent | 8,733 | 3.7 | 61 | <26; ≥26 to <30; ≥30 to <35; ≥35 |
All‐cause mortality Cardiovascular mortality |
| Zoppini et al.32 | 2003 | Type 2 diabetes | Prevalent | 3,398 | 10 | 66 |
≤25.4; 25.5–27.9; 28.0–30.9; ≥30.9 (<65 years) ≤24.6; 24.7–26.9; 27.0–29.8; ≥29.9 (≥65 years) |
All‐cause mortality Cardiovascular mortality |
CVD, cardiovascular disease.
The results of the present meta‐analysis showed a trend toward a lower risk of all‐cause mortality for obese patients as compared with normal weight patients, but it did not attain statistical significance (HR 0.87, 95% CI: 0.75–1.01, P = 0.058, I 2 = 91.8%; Figure 2a). Significant heterogeneities were observed among studies. Sensitivity analysis showed that no individual study unduly influenced the effect estimates. To further investigate the heterogeneity among studies, subgroup analysis was carried out, and it showed that the significantly lower risk of mortality was only observed in the elderly patients (HR 0.69, 95% CI: 0.63–0.75, P < 0.0001, I 2 = 50.4%), as well as in studies with <10 years of clinical follow up (HR 0.78, 95% CI: 0.65–0.93, P = 0.0006, I 2 = 85.5%), but not observed for younger patients and studies with >10 years of follow‐up (Table 2). In addition, meta‐regression analyses also showed that the survival benefit for obese patients was more pronounced along with the increased age, whereas it attenuated as the follow‐up duration increased. In obese patients, HR of all‐cause mortality decreased by 2.5% with increased per‐year of age (P = 0.018), whereas it increased by 3.2% with increased per‐year of follow‐up (P = 0.010; Figure 3). No significant modification was observed for the number of patients, number of mortality events, incident or prevalent diabetes and standard or non‐standard BMI classifications during meta‐regression analysis. No significant publication bias was observed by funnel plots (Begg's test: P = 0.88, funnel plot in Figure 4).
Figure 2.
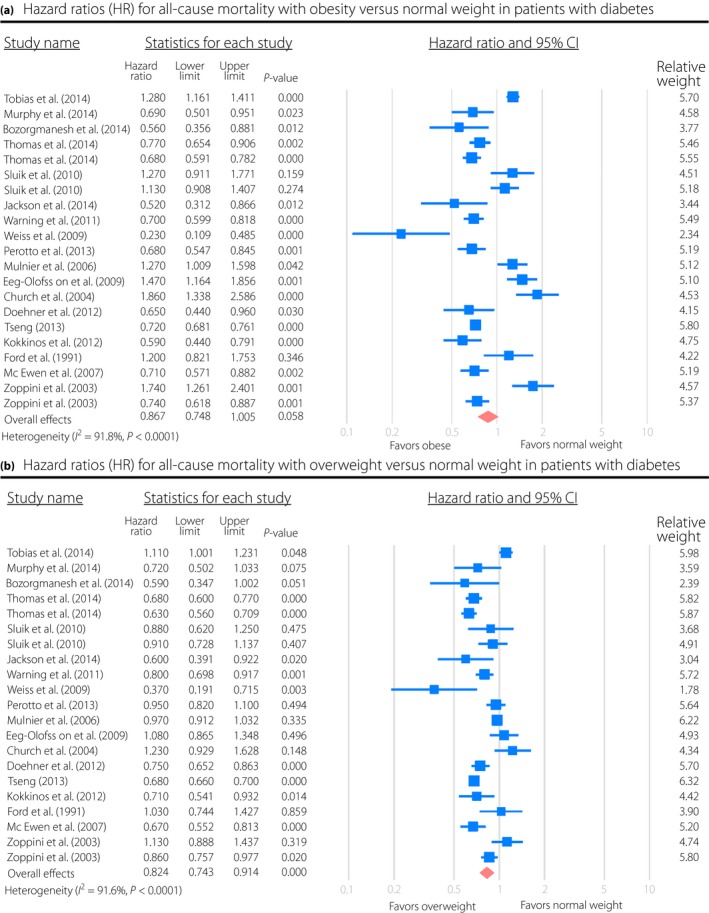
Hazard ratios (HR) for (a) all‐cause mortality with obesity or (b) overweight vs normal weight in patients with diabetes. CI, confidence interval.
Table 2.
Subgroup analyses of overweight and obesity, and the risk of all‐cause mortality in patients with diabetes
| Separate analysis | No. studies | Hazard ratios (95% CI) | P‐value | Heterogeneity, I 2 (%) | Heterogeneity P‐value |
|---|---|---|---|---|---|
| Overweight vs normal weight | |||||
| Elderly | 6 | 0.78 (0.73–0.84) | 0.001 | 69.7% | 0.005 |
| Non‐elderly | 5 | 0.83 (0.72–0.96) | 0.009 | 67.2% | 0.016 |
| Female | 3 | 0.87 (0.64–1.19) | 0.40 | 93.9% | <0.0001 |
| Male | 3 | 0.88 (0.58–1.32) | 0.54 | 96.2% | <0.0001 |
| No. patients ≥5,000 | 8 | 0.81 (0.70–0.94) | 0.006 | 95.2% | <0.0001 |
| No. patients <5,000 | 10 | 0.85 (0.75–0.96) | 0.012 | 65.4% | 0.001 |
| Follow‐up duration (≥10 years) | 6 | 0.97 (0.78–1.21) | 0.78 | 95.5% | <0.0001 |
| Follow‐up duration (<10 years) | 12 | 0.76 (0.67–0.85) | <0.0001 | 83.6% | <0.0001 |
| Incident diabetes | 4 | 0.75 (0.54–1.04) | 0.08 | 95.1% | <0.0001 |
| Non‐incident diabetes | 14 | 0.84 (0.75–0.94) | 0.003 | 90.7% | <0.0001 |
| Standard BMI category | 12 | 0.82 (0.72–0.93) | 0.002 | 93.6% | <0.0001 |
| Non‐standard BMI category | 6 | 0.83 (0.70–0.99) | 0.041 | 73.3% | 0.001 |
| Non‐smoking | 4 | 0.81 (0.53–1.23) | 0.33 | 76.3% | 0.005 |
| Obese vs normal weight | |||||
| Elderly | 6 | 0.69 (0.63–0.75) | <0.0001 | 50.4% | 0.073 |
| Non‐elderly | 5 | 1.01 (0.84–1.20) | 0.96 | 80.1% | 0.008 |
| Female | 3 | 1.06 (0.67–1.67) | 0.80 | 97.3% | <0.0001 |
| Male | 3 | 0.97 (0.65–1.46) | 0.88 | 94.7% | <0.0001 |
| No. patients ≥5,000 | 8 | 0.95 (0.77–1.16) | 0.61 | 94.5% | <0.0001 |
| No. patients <5,000 | 10 | 0.78 (0.61–1.01) | 0.058 | 87.2% | <0.0001 |
| Follow‐up duration (≥10 years) | 6 | 1.07 (0.78–1.42) | 0.67 | 96.0% | <0.0001 |
| Follow‐up duration (<10 years) | 12 | 0.78 (0.65–0.93) | 0.006 | 85.5% | <0.0001 |
| Incident diabetes | 4 | 0.80 (0.55–1.18) | 0.26 | 95.7% | <0.0001 |
| Non‐incident diabetes | 14 | 0.88 (0.75–1.04) | 0.14 | 89.3% | <0.0001 |
| Standard BMI category | 12 | 0.91 (0.76–1.09) | 0.30 | 93.4% | <0.0001 |
| Non‐standard BMI category | 6 | 0.77 (0.57–1.04) | 0.087 | 86.3% | <0.0001 |
| Non‐smoking | 5 | 0.77 (0.50–1.20) | 0.25 | 99.7% | <0.0001 |
BMI, body mass index.
Figure 3.
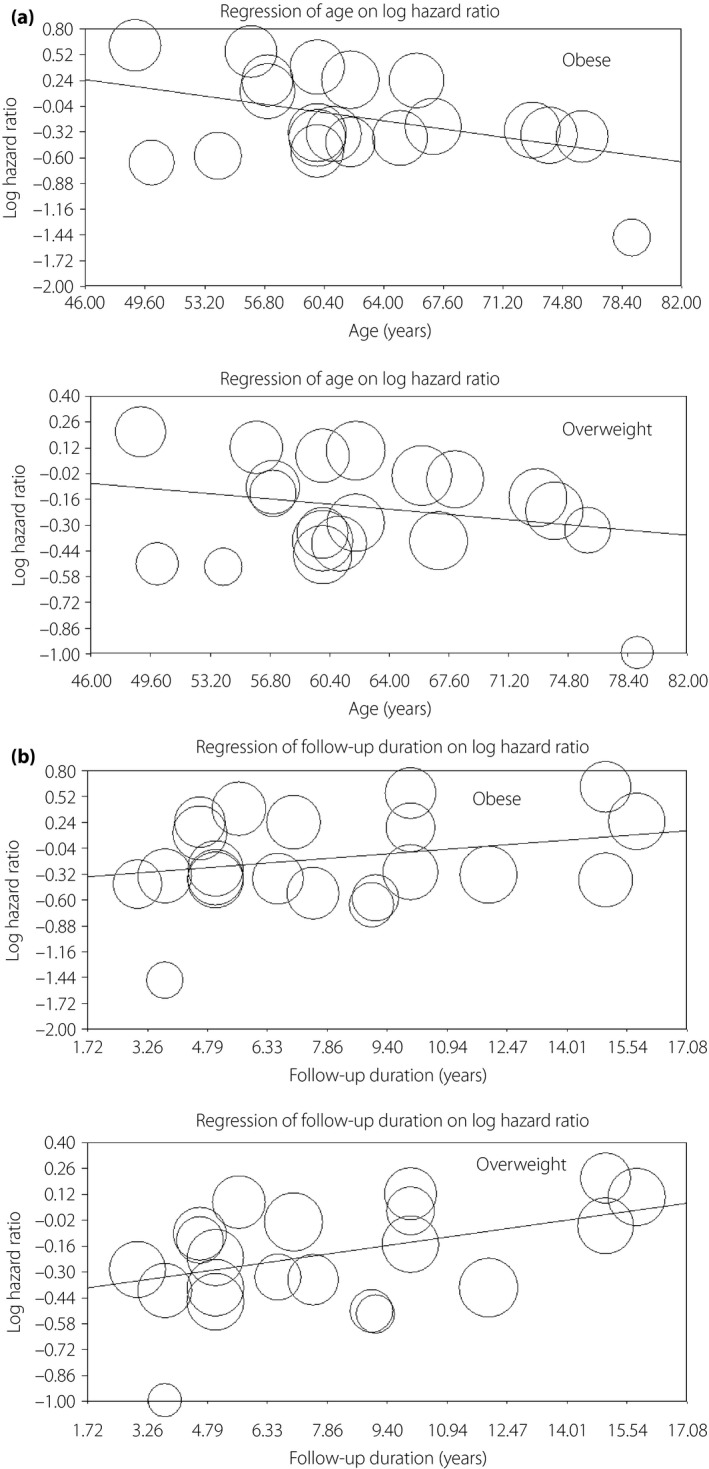
Meta‐regression on hazard ratios of all‐cause mortality for (a) age, as well as (b) follow‐up duration in overweight and obese patients vs normal weight patients (the size of the circles represents the individual study weights). (a) The meta‐regression was carried out among all studies with estimates of all‐cause mortality with the mean age of each study. P for obesity = 0.018, coefficient = 0.025; P for overweight = 0.31, coefficient = 0.008. (b) The meta‐regression was carried out among all studies with estimates of all‐cause mortality with the follow‐up duration of each study. P for obesity = 0.010, coefficient = 0.032; P for overweight = 0.041, coefficient = 0.030.
Figure 4.
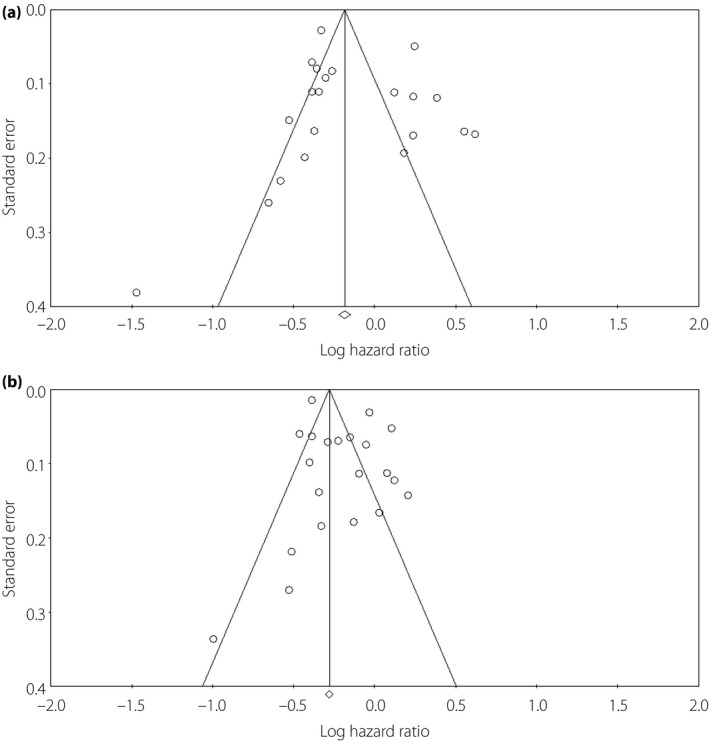
Funnel plot based on log hazard ratio of all‐cause mortality with (a) obese and (b) overweight patients vs normal weight patients. Begg's test: P = 0.88 for obese; Begg's test: P = 0.53 for overweight vs normal weight patients.
In terms of overweight patients, there was a significantly reduced risk of all‐cause mortality in comparison with normal weight patients (HR 0.82, 95% CI: 0.74–0.91, P < 0.0001, I 2 = 91.6%; Figure 2b). Sensitivity analysis showed that no individual study unduly influenced the estimates. Separate analysis showed that the results were consistent across trials except for the follow‐up duration (Table 2). The survival benefits of being overweight disappeared among studies with >10 years of clinical follow up (HR 0.97, 95% CI: 0.78–1.21, P = 0.78, I 2 = 95.5%). Meta‐regression analysis also confirmed this finding, and it showed no modification of the estimated effect sizes assessed by the number of patients, number of mortality events, mean age, incident or prevalent diabetes and standard or non‐standard BMI classifications. However, HRs of all‐cause mortality were raised by 3.0% (P = 0.041) as per‐year of follow‐up duration increased (Figure 3b). There was no apparent systematic bias as estimated by funnel plots (Begg's test: P = 0.53, funnel plot in Figure 4). In further analysis, we included the two studies10, 19 evaluating the risk of mortality for overweight/obesity vs normal weight patients, and the results of the meta‐analysis remained consistent (HR 0.84, 95% CI: 0.77–0.91, P < 0.0001, I 2 = 91.5%).
There were five studies22, 26, 31, 32, 33, with 19,643 patients, which evaluated the relationship of BMI and cardiovascular mortality for patients with diabetes. Meta‐analysis of these studies showed that overweight patients were associated with 15% reduced risks of cardiovascular mortality compared with those who were normal weight (HR 0.85, 95% CI: 0.74–0.97, P = 0.015, I 2 = 12.9%). However, no significant difference was found for the risk of cardiovascular mortality between obese and normal weight patients (HR 0.95, 95% CI: 0.70–1.28, P = 0.72, I 2 = 69.4%).
Discussion
Although the link between obesity and mortality has been extensively investigated in a variety of clinical conditions, the prognostic value of obesity for patients with type 2 diabetes remains controversial. The present meta‐analysis represents the largest data on this topic, including 20 studies of >250,000 individuals, and the results of the present study showed a significantly lower risk of mortality in overweight patients compared with normal weight patients; however, the survival benefits were attenuated with longer follow‐up durations. In addition, the beneficial prognostic impact of obesity was only observed among the elderly patients, whereas the discrepancy on age was not found among overweight patients.
The inverse relationship between BMI and mortality as shown in the present study is generally consistent with a prior meta‐analysis carried out by Liu et al.5, which enrolled nine studies and 161,984 participants. In their study, the relative risks (RRs) of all‐cause mortality in overweight (RR 0.81, 95% CI: 0.74–0.90) and obese (RR 0.72, 95% CI: 0.63–0.81) patients with diabetes were also significantly reduced compared with the normal or non‐overweight patients. However, some studies only reported the hazard ratio of BMI on mortality, but did not report the events rate29, 30, 31, 32, 33, and therefore these studies were not included in the prior meta‐analysis. However, the present meta‐analysis included all these studies, and we found a wide range of follow‐up durations for these enrolled studies. Therefore, we also carried out meta‐regression analyses to further investigate the heterogeneity of studies, and we found that the lower risk of mortality for overweight or obesity attenuated with longer follow‐up durations. To avoid unintentional or intentional weight loss secondary to diabetes development and diagnosis, several recent studies only enrolled patients with incident diabetes, and they found the inverse relationship of BMI and mortality also existed for incident diabetes10, 12. The results from the present meta‐analysis confirmed this finding. Separate analysis showed that the survival benefits of overweight remained consistent irrespective of incident or prevalent diabetes (Table 2).
Notably, subgroup as well as meta‐regression analysis in the present study showed that the survival benefits of obesity were more pronounced among elderly patients. In fact, it has been reported by some prior studies. Zoppini et al.33 investigated 3,398 patients with type 2 diabetes, followed up for 10 years, and 1,212 deaths occurred during the follow‐up period. They found that the obesity paradox was only observed among elderly patients, but not for those younger patients (aged <65 years). Additionally, there were two studies (Weiss et al.24 and Murphy et al.11) that only enrolled elderly patients with diabetes. Weiss et al.24 showed that BMI was inversely associated with all‐cause (RR 0.89, 95% CI: 0.83–0.96, P = 0.002) and cardiovascular mortality (RR 0.83, 95% CI: 0.74–0.93, P = 0.002) among hospitalized elderly patients with type 2 diabetes. Murphy et al.11 compared the mortality of obesity and normal weight with overweight in the elderly patients with diabetes. They used overweight as the reference, and found an increased risk of mortality among normal weight compared with overweight participants (HR 1.72, 95% CI: 1.12–2.64). One possible explanation is that obesity usually clusters with several cardiovascular risk factors, such as dyslipidemia, hypertension and so on, and these disorders tend to be more frequently developed in elderly patients, even without overweight or obesity33, 35. However, in younger patients, these disorders are less frequently observed, and their existence might not be considered as mere confounders, but possible intermediate mechanisms of the obesity‐related damage, and therefore the detrimental effects of these disorders might be more apparent among younger patients33. Additionally, in younger patients, other factors, such as genetic background, latent autoimmune diabetes in adults and so on might play an important role in the development of diabetes, which might link to poorer prognosis. Third, previous studies suggest muscle mass is inversely associated with insulin resistance10, 11, 36, 37, and age‐related loss of lean muscle mass could result in a lower bodyweight in elderly patients, and thus contribute to the worse outcome.
Another interesting finding of the present study was the late ‘catch‐up’ phenomenon. We found the survival benefits of overweight and obesity were time‐dependent, which were attenuated with time, and eventually, disappeared after 10 years of follow‐up. A possible explanation for this decrescendo effect might be related to the underlying chronic disease or frailty, both of which can cause weight loss and elevate the risk of death13, and studies with short follow‐up duration are more likely to be affected by this problem.
Several limitations of this meta‐analysis need to be addressed. First, BMI suffers from the inability to discriminate body fat from lean body mass. Second, individual patient data were not available for the meta‐analysis, and therefore, there might be potential variables that cannot be adjusted across studies. Third, the majority of existing studies only provided baseline BMI values, so the influence of weight change during the follow‐up period cannot be taken into account. Fourth, standard BMI classifications were not used by some studies, although we carried out a separate analysis for studies with or without standard classification, it might still introduce bias into the results. Fifth, substantial heterogeneity was found in the present meta‐analysis; although several attempts have been made to investigate the sources of heterogeneity through various sensitivity analyses and meta‐regression, we did not find a simple explanation or method to account for this variability. The inconsistency of follow‐up duration across individual studies can partly explain the heterogeneity, but a high level of heterogeneity still exists within different subgroup analyses. Additionally, 6 out of 20 studies in the meta‐analysis were unable to determine whether participants had type 2 diabetes or other less common forms of diabetes in adults. However, because the studies were carried out in adults where the vast majority (>95%) of diabetes can be assumed to be type 2, the findings should apply to persons with type 2 diabetes2. Finally, only BMI was evaluated in the present analysis. Other modalities associated with bodyweight, including body composition and fitness11, were not included because of insufficient data on these parameters.
In conclusion, the results from the present meta‐analysis showed a lower risk of mortality in overweight/obese patients with diabetes compared with normal weight patients, and the beneficial prognostic impact of obesity was more pronounced among elderly patients, but attenuated with longer follow‐up durations. However, caution should be taken in interpreting the results, as the design of the present study did not permit any verification of the causal relationship between bodyweight and prognosis in patients with diabetes. To definitively answer this question, prospective randomized controlled studies assessing the survival benefits of supervised weight‐control programs across different BMI categories in patients with diabetes are urgently required.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 Quality assessment of included studies.
J Diabetes Investig 2018; 9: 44–54
References
- 1. Narayan KM, Boyle JP, Thompson TJ, et al Lifetime Risk for Diabetes Mellitus in the United States. JAMA 2003; 290: 1884–1890. [DOI] [PubMed] [Google Scholar]
- 2. Carnethon MR1, Rasmussen‐Torvik LJ, Palaniappan L. The obesity paradox in diabetes. Curr Cardiol Rep 2014; 16: 446. [DOI] [PubMed] [Google Scholar]
- 3. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37: 1595–1607. [DOI] [PubMed] [Google Scholar]
- 4. Bantle JP, Wylie‐Rosett J, Albright AL, et al Nutrition recommendations and interventions for diabetes: a position statement of the American diabetes association. Diabetes Care 2008; 31: S61–S78. [DOI] [PubMed] [Google Scholar]
- 5. Liu XM, Liu YJ, Zhan J, et al Overweight, obesity and risk of all‐cause and cardiovascular mortality in patients with type 2 diabetes mellitus: a dose‐response meta‐analysis of prospective cohort studies. Eur J Epidemiol 2015; 30: 35–45. [DOI] [PubMed] [Google Scholar]
- 6. Angerås O, Albertsson P, Råmunddal T, et al Evidence for obesity paradox in patients with acute coronary syndromes: a report Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J 2013; 34: 345–353. [DOI] [PubMed] [Google Scholar]
- 7. Wang ZJ, Zhou YJ, Zhao YX, et al Effect of obesity on repeat revascularization in patients undergoing percutaneous coronary intervention with drug‐eluting stents. Obesity (Silver Spring) 2012; 20: 141–146. [DOI] [PubMed] [Google Scholar]
- 8. Padwal R, McAlister FA, McMurray JJ, et al Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC). The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta‐analysis of individual patient data. Int J Obes (Lond) 2014; 38: 1110–1114. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt D, Salahudeen A. The obesity‐survival paradox in hemodialysis patients: why do overweight hemodialysis patients live longer? Nutr Clin Pract 2007; 22: 11–15. [DOI] [PubMed] [Google Scholar]
- 10. Carnethon MR, De Chavez PJ, Biggs ML, et al Association of weight status with mortality in adults with incident diabetes. JAMA 2012; 308: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murphy RA, Reinders I, Garcia ME, et al Age, Gene/Environment Susceptibility‐Reykjavik Study (AGES‐Reykjavik). Adipose tissue, muscle, and function: potential mediators of associations between body weight and mortality in older adults with type 2 diabetes. Diabetes Care 2014; 37: 3213–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas G, Khunti K, Curcin V, et al Obesity paradox in people newly diagnosed with type 2 diabetes with and without prior cardiovascular disease. Diabetes Obes Metab 2014; 16: 317–325. [DOI] [PubMed] [Google Scholar]
- 13. Tobias DK, Pan A, Jackson CL, et al Body‐mass index and mortality among adults with incident type 2 diabetes. N Engl J Med 2014; 370: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bozorgmanesh M, Arshi B, Sheikholeslami F, et al No Obesity Paradox‐BMI Incapable of Adequately Capturing the Relation of Obesity with All‐Cause Mortality: an Inception Diabetes Cohort Study. Int J Endocrinol 2014; 2014: 282089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stroup DF, Berlin JA, Morton SC, et al Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 16. Global database on body mass index . Body mass index classification of WHO. Available from: http://wwwwhoint/bmi/indexjsp?introPage=intro_3html Accessed May 6, 2015.
- 17. Wells GA, Shea B, O'Connell D, et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2011. Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.asp accessed May 6, 2015.
- 18. Higgins JP, Thompson SG, Deeks JJ, et al Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 20. Lajous M, Bijon A, Fagherazzi G, et al Body mass index, diabetes, and mortality in French women: explaining away a “paradox”. Epidemiology 2014; 25: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waring ME, Saczynski JS, McManus D, et al Weight and mortality following heart failure hospitalization among diabetic patients. Am J Med 2011; 124: 834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perotto M, Panero F, Gruden G, et al Obesity is associated with lower mortality risk in elderly diabetic subjects: the Casale Monferrato study. Acta Diabetol 2013; 50: 563–568. [DOI] [PubMed] [Google Scholar]
- 23. Eeg‐Olofsson K, Cederholm J, Nilsson PM, et al Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52: 65–73. [DOI] [PubMed] [Google Scholar]
- 24. Weiss A, Boaz M, Beloosesky Y, et al Body mass index and risk of all‐cause and cardiovascular mortality in hospitalized elderly patients with diabetes mellitus. Diabet Med 2009; 26: 253–259. [DOI] [PubMed] [Google Scholar]
- 25. Tseng CH. Obesity paradox: differential effects on cancer and noncancer mortality in patients with type 2 diabetes mellitus. Atherosclerosis 2013; 226: 186–192. [DOI] [PubMed] [Google Scholar]
- 26. Sluik D, Boeing H, Montonen J, et al Associations Between General and Abdominal Adiposity and Mortality in Individuals With Diabetes Mellitus. Am J Epidemiol 2011; 174: 22–34. [DOI] [PubMed] [Google Scholar]
- 27. Mulnier HE, Seaman HE, Raleigh VS, et al Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23: 516–521. [DOI] [PubMed] [Google Scholar]
- 28. Church TS, Cheng YJ, Earnest CP, et al Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004; 27: 83–88. [DOI] [PubMed] [Google Scholar]
- 29. Doehner W, Erdmann E, Cairns R, et al Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co‐morbidity: an analysis of the PROactive study population. Int J Cardiol 2012; 162: 20–26. [DOI] [PubMed] [Google Scholar]
- 30. Kokkinos P, Myers J, Faselis C, et al BMI–Mortality Paradox and Fitness in African American and Caucasian Men With Type 2 Diabetes. Diabetes Care 2012; 35: 1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ford ES, DeStefano F. Risk factors for mortality from all causes and from coronary heart disease among persons with diabetes. Findings from the National Health and Nutrition Examination Survey I Epidemiologic Follow‐up Study. Am J Epidemiol 1991; 133: 1220–1230. [DOI] [PubMed] [Google Scholar]
- 32. McEwen LN, Kim C, Karter AJ, et al Risk Factors for Mortality Among Patients With Diabetes: the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 2007; 30: 1736–1741. [DOI] [PubMed] [Google Scholar]
- 33. Zoppini G, Verlato G, Leuzinger C, et al Body mass index and the risk of mortality in type II diabetic patients from Verona. Int J Obes Relat Metab Disord 2003; 27: 281–285. [DOI] [PubMed] [Google Scholar]
- 34. Jackson CL, Yeh HC, Szklo M, et al Body mass index and all cause mortality in US adults with and without diabetes. J Gen Intern Med 2014; 29: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krauss RM, Wiston M, Fletcher BJ, et al Obesity. Impact on cardiovascular disease. Circulation 1998; 98: 1472–1476. [PubMed] [Google Scholar]
- 36. Folsom AR, Rasmussen ML, Chambless LE, et al The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. Diabetes Care 1999; 22: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 37. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 2011; 96: 2898–2903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Quality assessment of included studies.


