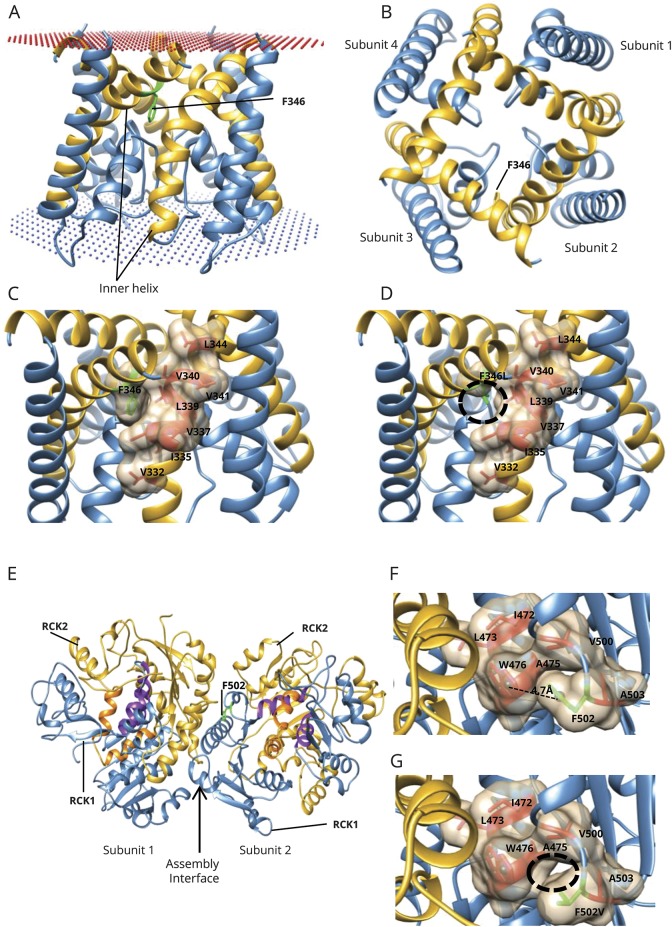
Figure 1. Modeling the ion channel and gating apparatus of KCNT1.
(A) Side view of the homology model of the KCNT1 ion channel (residues 278–346) as a tetramer. F346 is present on the edge of the inner helix (in gold) and interacts with the inner helix of the adjacent subunit in the tetrameric arrangement. Membrane position is shown in spheres. (B) Top view of the tetramer arrangement of the ion channel and location of F346 on the inner helix. (C) F346 is part of the hydrophobic cavity (shown as surface), which mediates interactions between the inner membrane helices of the 2 subunits. F346 is shown in green; the surrounding hydrophobic residues are shown in red. (D) On mutation to leucine (F346L, in green), the hydrophobic interactions between the 2 subunits are likely to be reduced (black circle) because the side chain of leucine is much shorter than phenylalanine. (E) Model of a dimer of the gating ring (residues 373–1,044; residues 1,045–1,174 could not be modeled), which is a tetramer (dimer of the modeled dimer). Each subunit possesses 2 RCK domains: RCK1 (in blue) and RCK2 (in gold). F502 (in green) is present in the RCK1 domain, near the intersubunit interface (assembly interface). The RCK1-RCK2 intrasubunit interface is purple (residues from RCK1) and orange (residues from RCK2). The dimer interfaces formed by both RCK-1 and RCK-2 are indicated by an arrow. (F) F502 (green) and its neighboring hydrophobic residues (red), including W476, with which it could potentially form a pi-pi interaction. Distance between the centroid (spheres) of the 2 rings (F502 and W476) is 4.7 Å, and the angle between the ring planes is 27.3°. (G) F502V could abolish the formation of the potential pi-pi interaction with W476 and is likely to reduce the hydrophobic interactions (black circle) because the side chain of valine is smaller than that of phenylalanine.