Figure 3.
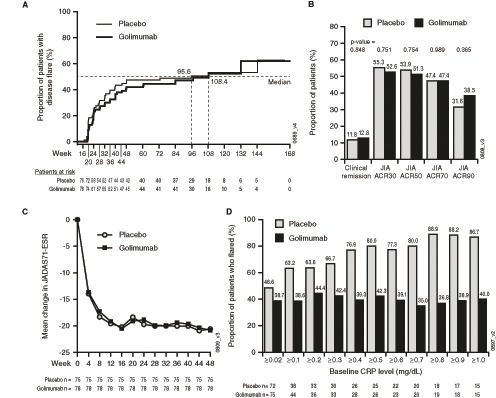
(A) Kaplan-Meier plot of JIA flare events starting Part 2 of the study. (B) Proportions of randomised patients with a JIA ACR30/50/70/90 response and clinical remission at week 48 as compared with baseline (week 0). (C) Mean change in JADAS71-ESR through week 48. (D) The proportion of patients who flared during the trial. Flare rates remained relatively stable over time among patients who continued with golimumab after week 16 and remained relatively similar regardless of the baseline CRP levels. However, among patients receiving placebo in Part 2, the proportion of patients who flared in Part 2 increased depending on CRP levels at baseline: for example, for patients with baseline CRP levels ≥0.02 mg/dL, the flare rate was 48.6% (35/72) and for those with baseline CRP levels ≥1.0 mg/dL, the flare rate was 86.7% (13/15). JIA ACR30/50/70/90, ≥30%/50%/70%/90% improvement in the American College of Rheumatology juvenile idiopathic arthritis response criteria; CRP, C reactive protein; JADAS71-ESR, Juvenile Arthritis Disease Activity Score using erythrocyte sedimentation rate; JIA, juvenile idiopathic arthritis.
