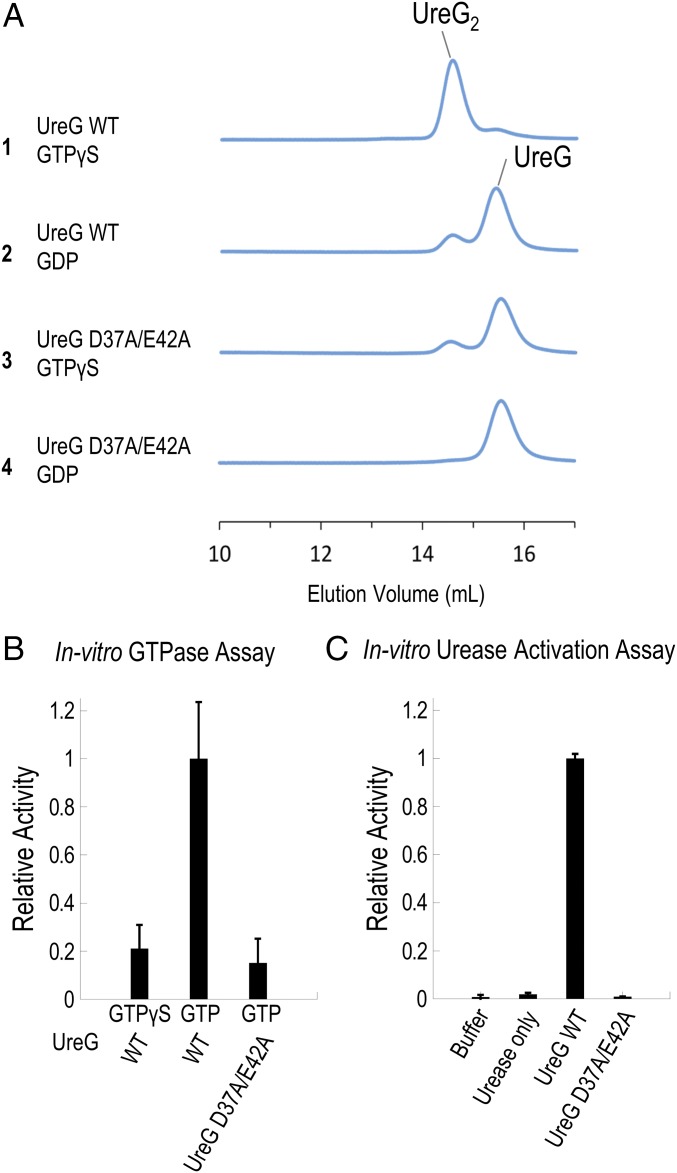
Fig. 3.
Double mutation D37A/E42A abolishes the formation of the nickel-charged HpUreG dimer, GTPase activity, and urease activation. (A) Protein samples of 30 µM H. pylori UreG (WT or mutant) were mixed with 45 µM nickel ion and 300 µM GTPγS/GDP and were analyzed using SEC/SLS. The wild-type UreG mainly existed as a dimer in the presence of GTPγS (injection 1), but as a monomer in the presence of GDP (injection 2). In contrast, the D37A/E42A mutant mainly existed as a monomer regardless of addition of GTPγS or GDP (injections 3 and 4). (B) GTP hydrolysis was followed by the amount of phosphate released using the malachite green assay as described in Materials and Methods. A total of 5 µM of UreG (WT or D37A/E42A mutant) was incubated with 300 µM of GTP/GTPγS in 2 mM MgSO4, 10 mM potassium bicarbonate, 4 µM NiSO4, 200 mM NaCl, 1 mM TCEP, 20 mM Hepes pH 7.5 buffer at 37 °C for 60 min. The hydrolysis rates were determined and analyzed by linear regression using the PRISM program (GraphPad Software). The hydrolysis rate of the wild-type HpUreG was significantly different from those of the double mutant and the GTPγS control (P < 0.01), while there were no significant differences between the mutant and the GTPγS control. Moreover, the slope of the regression lines for the double mutant and the GTPγS were not significantly deviated from zero. Relative activity was normalized using the hydrolysis rate of wild-type HpUreG (43 ± 7 nM phosphate/µM UreG/min). (C) A total of 10 µM apourease was activated by 40 µM UreG (WT or mutant) and 20 µM UreF2H2 complex in 2 mM MgSO4, 10 mM potassium bicarbonate, 45 µM NiSO4, 300 µM GTP, and 20 mM Hepes pH 7.5, 200 mM NaCl, 1 mM TCEP, at 37 °C for 20 min. Urease activity was determined by measuring the amount of ammonia released. In the “urease only” control, no urease accessory protein was added, while in the “buffer” control, no apourease or urease accessory proteins were added. Relative activity was normalized using the activity of urease activated by wild-type HpUreG (304 ± 5 µmol NH3/mg urease/min).