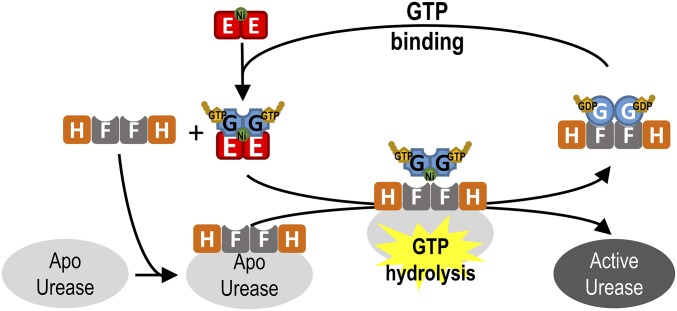
Fig. 6.
How conformational changes in UreG during the GTP hydrolysis/binding cycle facilitate urease maturation. GTP binding induces conformational changes in UreG that destabilize the UreG2F2H2 complex, causing UreG to dissociate from the complex and form the UreE2G2 complex with the nickel-charged UreE dimer. After receiving its nickel within the UreE2G2 complex, the nickel-charged UreG dimer is recruited to form the activation complex with apourease and UreF2H2. GTP hydrolysis induces conformational changes in the CPH motif of UreG, disrupting the square-planar coordination by Cys66/His68, and, hence, promotes release of the nickel ion for urease maturation. UreG, now in its GDP-bound state, prefers to form the UreG2F2H2 complex, which is now ready to receive its nickel from UreE2/Ni for another round of urease maturation.