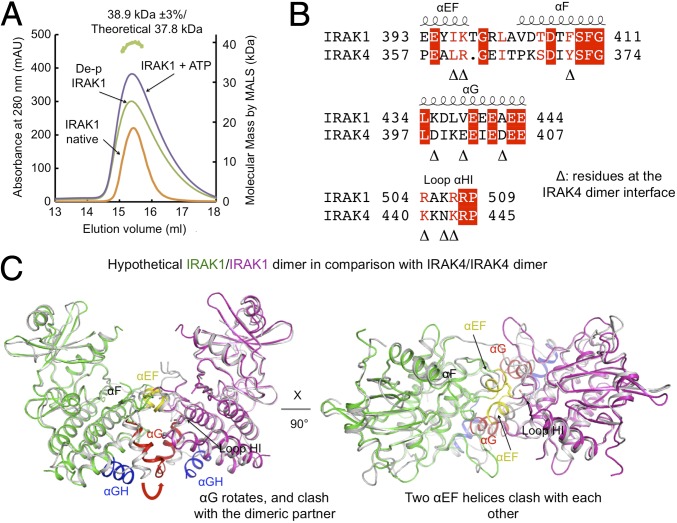
Fig. 5.
IRAK1 kinase domain is a monomer in solution in both phosphorylated and unphosphorylated states. (A) Gel filtration profiles and multiangle light scattering measurement showed that the IRAK1 kinase domain is a monomer in different states, different from the dimeric state of the IRAK4 kinase domain in the unphosphorylated form. The green track on the top marks the measured molecular mass of the dephosphorylated IRAK1 kinase domain. mAU, absorption units. (B) Sequence alignment between IRAK1 and IRAK4 at the IRAK4 dimerization interface. Important IRAK4 dimerization residues are indicated by triangles. (C) A model of a hypothetical IRAK1 homodimer would have caused clash in several dimerization elements including the αG and the αEF regions.