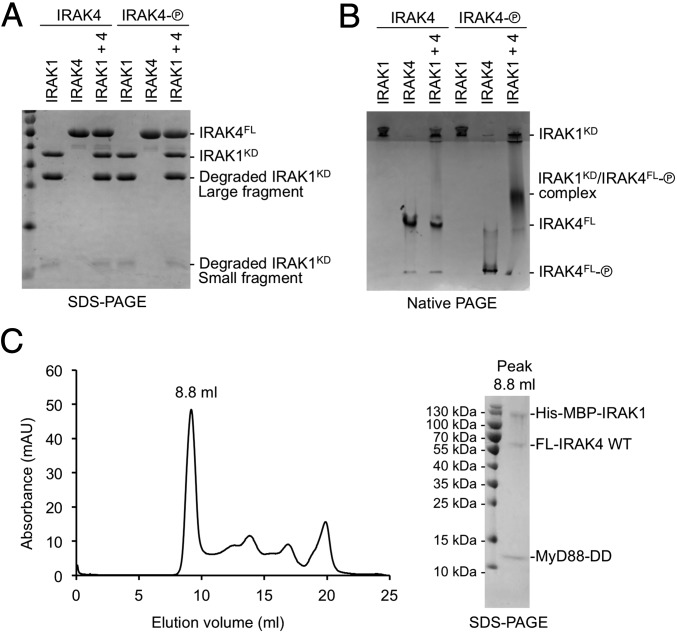
Fig. 6.
IRAK1 interacts weakly with phosphorylated IRAK4. (A) IRAK1 and two forms of IRAK4, unphosphorylated (left lanes, “IRAK4”) and phosphorylated (shown as an encircled P; right lanes, “IRAK4-P”) on an SDS-PAGE. (B) IRAK1 and two forms of IRAK4, unphosphorylated (left lanes, “IRAK4”) and phosphorylated (right lanes, “IRAK4-P”) on a native PAGE, showing the shifted band containing IRAK1 and phosphorylated IRAK4. (C) Gel filtration profile (Left) and SDS-PAGE (Right) of the peak fraction for the reconstituted complex of the MyD88 death domain (DD), full-length (FL) IRAK4, and His-MBP–tagged IRAK1 containing the DD and the kinase domain.