Thirteen years ago, microscopy researcher Volker Brinkmann spotted a bizarre behavior while peering at immune cells called neutrophils to study how they killed bacteria. Like the quick flick of a frog’s tongue, neutrophils shot out strands of nuclear DNA to trap passing microbes.
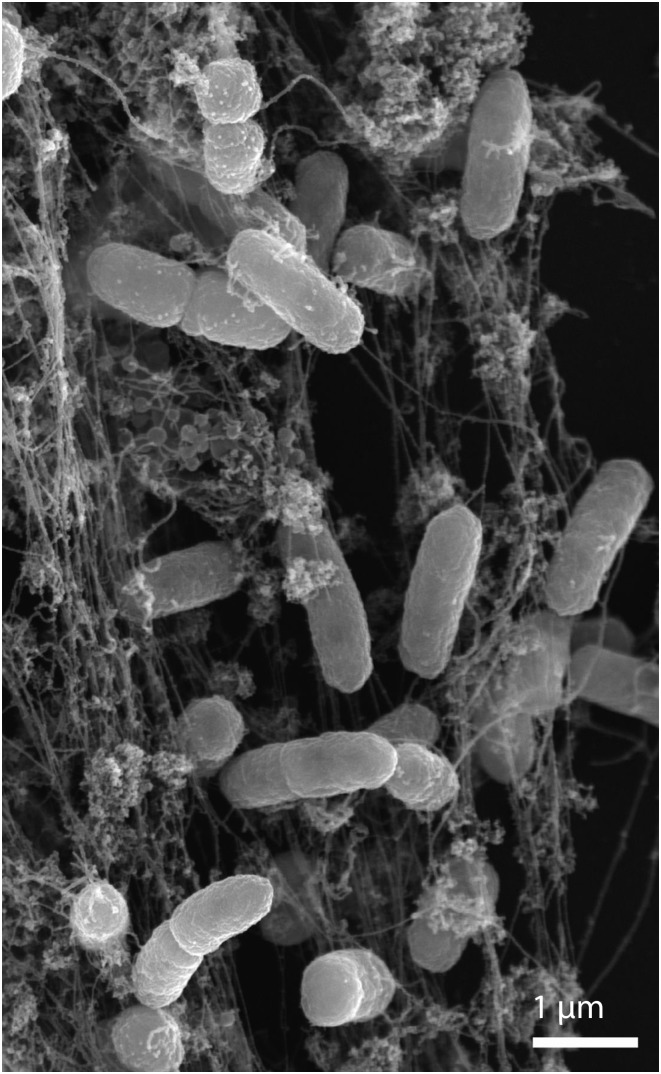
In this scanning electron microscope image, NETs trap human neutrophils incubated with Salmonella. Republished with permission of Rockefeller University Press, from ref. 9; permission conveyed through Copyright Clearance Center, Inc.
The act resulted in a web of genetic material, immune proteins, and dead bacteria that Brinkmann, immunologist Arturo Zychlinsky, and their colleagues at the Max Planck Institute for Infection Biology dubbed neutrophil extracellular traps (NETs) (1). DNA is a sticky molecule that can bind both human proteins and those that coat pathogens, and within cells DNA is arranged in chromatin along with acidic proteins known as histones, which can also act as potent antimicrobials. “My hypothesis is that eukaryotic chromatin evolved with this dual function of maintaining the integrity of the genome and defending the organism,” Zychlinsky says.
At first the idea that neutrophils might deploy genetic material as an immune arsenal was tough to stomach. “Before they proposed this mechanism, it would have been difficult to say that DNA had anything to do with fighting infections,” says immunologist Paul Kubes of the University of Calgary.
But since discovering these extracellular traps (ETs) in 2004, researchers have them found strewn across the tree of life. They’ve been reported in samples from chickens, plants, slime molds, insects, and other animals (2). In the human body, many kinds of immune cells catapult out their DNA; NETs are made in arthritic inflammation, cancer, diabetes, and other diseases.
Even so, Zychlinsky’s idea of DNA as a defense tool has been difficult to prove. Despite an apparent abundance of NETs, researchers haven’t yet uncovered a specific gene or pathway responsible for their formation. And the sheer diversity of ways in which NETs form has prompted researchers to question which ones serve some function (3). Are all of these snares purposefully laid, or are some simply experimental artefacts formed when cells die and release their contents?
“Like any other scientific field, the initial discovery generates a huge amount of enthusiasm and people saw NETs everywhere,” Kubes says. “I think we’ve passed that point, and now people are starting to question under what conditions NETs are formed more rigorously.”
Die Another Way
The first reports of NETs suggested that making these structures was a unique form of cell death. Typically, neutrophils kill pathogens via phagocytosis, literally eating the microbe and digesting it within an intracellular pocket filled with lethal enzymes. But cells that make NETs appear to spill these potent enzymes outside the cell in a process distinctly different from other forms of cell death, such as apoptosis or necrosis. “They start to dismantle their intracellular machinery, decorate the chromatin with enzymes like elastase and myeloperoxidase, and then they throw it out like a big fishing net,” says immunologist Helen Wright of the University of Liverpool.
In these early studies, neutrophils that were spurred into action by a chemical named phorbol myristate acetate (PMA) formed NETs in a suicidal explosion, killing both immune cells and microbes. Since then, other studies have revealed a surprising diversity of NETs and ways to make them.
One means of doing so entails a rapid burst. When neutrophils encounter certain microbes or microbial proteins such as PMA, they unravel their nuclear material until it fills up the cells, then dissolve intracellular membranes. Eventually they die, releasing their contents in a NET. But not all neutrophils die after releasing their DNA.
In 2013, Kubes and his colleagues reported that NET-forming cells could stay alive after tossing out their DNA in a process called “vital NETosis” (4). Although some neutrophils use mitochondrial DNA to make NETs, others release nuclear genetic material. Not all neutrophils use DNA as a weapon against microbes; some kill via phagocytosis, whereas others undergo NETosis. Right now, it isn’t clear how cells decide which way to die. Early data suggest that the age of a cell may play a role (5). Another study found that, in patients with multiple sclerosis, neutrophils from men were more likely to form NETs than those from women (6).
“For NET formation to be involved in such a range of diseases, it would need a mind-boggling number of signaling pathways and controls—or it's just a stochastic process and not controlled.”
—William Nauseef
And it’s not just microbes that trigger this DNA release. Even in the absence of pathogens—under sterile conditions—some neutrophils make NETs in autoimmune conditions such as lupus or arthritis in humans. Here, DNA traps autoantigens and exacerbates damaging inflammatory reactions (7).
This diversity in the contents of NETs and the ways they’re made makes some researchers skeptical of their supposed function. “Something that’s so diverse would need to have extraordinary modulation,” says William Nauseef of the University of Iowa Health Care. “For NET formation to be involved in such a range of diseases, it would need a mind-boggling number of signaling pathways and controls—or it’s just a stochastic process and not controlled.”
Although there’s no question that the data are real, Nauseef and others suggest that NETs are simply debris left after cells die during a skirmish—not the result of sophisticated cellular plans. “It’s never been any concern that people aren’t legitimate in their experimental design and what they do,” Nauseef says. “It’s just a difference in how we interpret the data.” Confusing matters, free-floating DNA—the backbone of NETs—is, for a variety of reasons, prolifically released when cells die.
The questions remain: Are NETs truly “by design” or simply accidental byproducts of DNA released when cells die? And if NETs are purposefully made, do cells produce them in vivo?
Looking Past DNA
Experimental approaches used to investigate NETs may have contributed to misleading results, some researchers assert. For starters, many of the early experiments with NETs were done with high concentrations of PMA—at levels that cells within the body are unlikely to ever encounter. “There’s no question that the treatment causes this explosion of cells, but PMA would never be in circulation at those concentrations,” he adds. “When you move away from PMA and look at physiological stimuli, you start seeing very different things.”
Plus, extending data from animal studies to human biology has been difficult. Neutrophils in culture die very easily, and mouse and human neutrophils behave very differently, explains Mariana Kaplan of the NIH’s National Institute of Arthritis and Musculoskeletal and Skin Diseases. “The science of neutrophil biology has lagged behind other immune cells,” she adds.
Simply identifying NETs has proven challenging. In human studies, the free-floating mesh of DNA and proteins that makes a NET can be hard to spot—or look surprisingly different—depending on the tissue or organ they’re present in. A NET made within a blood vessel, for example, could carry different proteins and be packed differently from one within a brain capillary.
Now, Kubes, Kaplan, and others are working to find clearer definitions of ETs. Researchers do routinely observe certain steps in the process of NET formation, such as the need for reactive oxygen species, chromatin decondensation, and dissolution of intracellular membranes before exterior ones. One recent study, for example, distinguishes NET formation from two other kinds of cell death in which DNA is released based on the activity of an enzyme named PAD4 (protein arginine deaminase 4), which is thought to be essential to make NETs (8). But many other aspects, such as whether specific subsets of neutrophils make NETs in different circumstances or the precise sequence of steps and proteins required, are still murky. “It’s going to be very difficult until there is a rigorous classification scheme that defines NETs biochemically and not morphologically,” Nauseef says.
To detect NETs, researchers often use a cellular stain that binds extracellular DNA but doesn’t enter cells. However, Kubes and others recommend that a better way to detect true ETs—and not just free-floating DNA—is to look for DNA, histones, and active enzymes secreted by neutrophils all in close proximity, as they’d occur in a NET. Fluorescence microscopy is the best way to find these structures, but even so, counting NETs can be “very time consuming and a little bit subjective,” Kaplan says.
Ultimately, the best proof that ETs are biologically relevant—and made purposefully—would be to find a specific gene or pathway that’s exclusively needed for NET formation, Zychlinsky says. It might also make them easier to study. But so far, such a mechanism has been elusive; although certain neutrophil genes are known to be needed to make NETs, they also serve other functions. For example, cells that lack a protein named NADPH oxidase can’t make NETs. But this enzyme is also needed for phagocytosis and other activities. “So far, we have discovered genes that are important for neutrophil function that are also relevant to NET formation,” Zychlinsky says. “We have not discovered a new NET gene.”
Seeking Clinical Significance
Until researchers better understand NETs and how they’re made, drugs that target DNA ETs to treat infections may be difficult to develop. But their more immediate relevance might be in human autoimmune disease, where ETs appear even in the absence of microbial triggers.
Whether this DNA is a reaction to an antigen or the trigger for autoimmunity is still a mystery. But data suggest that the proteins NETs bear vary with underlying stimuli and specific microenvironments. In rheumatoid arthritis, for example, Kaplan and her colleagues have found that different triggering proteins can alter the inflammatory proteins present on NETs. But—regardless of why they’re made—they appear to induce tissue damage. “In autoimmunity, the focus seems clearer: shutting down NET production could alleviate inflammatory symptoms,” Kaplan says.
Stopping NET formation in these conditions could reduce symptoms and may even stop disease progression. “The key aspect in moving forward with therapeutic interventions will be to see if other neutrophil functions remain when NET formation is diminished,” says Kaplan. “And for individuals who are more prone to infections or NET-impaired, can restoring NET formation improve their ability to fight microbes?”
Supplementary Material
References
- 1.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Goldmann O, Medina E. The expanding world of extracellular traps: Not only neutrophils but much more. Front Immunol. 2013;3:420. doi: 10.3389/fimmu.2012.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nauseef WM, Kubes P. Pondering neutrophil extracellular traps with healthy skepticism. Cell Microbiol. 2016;18:1349–1357. doi: 10.1111/cmi.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yipp BG, Kubes P. NETosis: How vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 5.Hazeldine J, et al. Impaired neutrophil extracellular trap formation: A novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13:690–698. doi: 10.1111/acel.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tillack K, et al. Gender differences in circulating levels of neutrophil extracellular traps in serum of multiple sclerosis patients. J Neuroimmunol. 2013;261:108–119. doi: 10.1016/j.jneuroim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23:279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 8.Konig MF, Andrade F. A critical reappraisal of neutrophil extracellular traps and NETosis mimics based on differential requirements for protein citrullination. Front Immunol. 2016;7:461. doi: 10.3389/fimmu.2016.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J Cell Biol. 2012;198:773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]