Abstract
The use of an achiral metal–organic framework for structure determination of chiral compounds is demonstrated for camphene and pinene. The structure of enantiopure β-pinene can be resolved using the crystalline sponge method. However, α-pinene cannot be resolved using enantiopure material alone because no ordering of guest molecules takes place in that case. Interestingly, enantiomeric pairs order inside the channels of the host framework when impure (+)-camphene is offered to the host, which is also the case when a racemic mixture of α-pinene is used. A mixture of (+)-α-pinene and (−)-β-pinene also leads to ordered incorporation in the host, showing the influence of the presence of an inversion center in the host framework. We further show that powder X-ray diffraction provides a direct view on incorporation of ordered guest molecules. This technique, therefore, provides a way to determine the optimal and/or minimal soaking time. In contrast, color change of the crystal only demonstrates guest uptake, not ordering. Moreover, we show that color change can also be caused by guest-induced host degradation.
Short abstract
Enantiomeric pairs of camphene in a crystalline sponge. The crystalline sponge method was used to determine the structures of camphene and pinene. The metal−organic framework host crystal selectively orders enantiomeric pairs for camphene and α-pinene. Although it was demonstrated by others that enantiopure compounds could be resolved using this method, this proved to be impossible for camphene and α-pinene.
Introduction
Single-crystal X-ray diffraction is the most effective way to obtain the absolute configuration of molecules. However, the preparation of well-defined crystals suitable for X-ray analysis can be a severe bottleneck. The “crystalline sponge method” was introduced by Fujita et al.1 for cases where conventional crystallization and subsequent structure elucidation proves difficult (e.g., in the case of liquids, oils, or plastic crystals). In the crystalline sponge method the compound of interest is included as a guest into a porous metal–organic framework (MOF), e.g., consisting of ZnI2 and 2,4,6-tri(pyridin-4-yl)-1,3,5-triazine,1,2 after which a single-crystal X-ray diffraction experiment can be performed. The term “crystalline sponge” was originally coined for clathrate systems, where a guest is crystallized in the presence of a porphyrin, yielding a cocrystal. Hundreds of molecular structures have thus been obtained using this clathrate system approach.3−7 Inclusion of molecular guests into MOFs during synthesis was already shown in the 1990s,8,9 but with the crystalline sponge method they can be included after MOF synthesis by simply soaking the MOF host crystal in a solution containing the guest. The possibility to elucidate molecular structures,10−22 as well as observing reaction mechanisms,23 and reaction intermediates24 makes the crystalline sponge method a unique tool for structure analysis, including absolute structure determination.
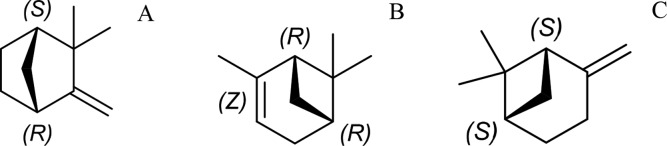
Molecular compounds are often chiral and the use of the crystalline sponge method for this class of materials is highly desirable. The porous framework introduced by Fujita and responsible for most of the crystalline sponge applications up to now has a space group that in principle is incompatible with chiral compounds. Nevertheless, successful structure determinations of several chiral compounds have been reported,2,25−27 but only after guest ordering resulted in the breaking of the inversion/mirror symmetry of the host–guest system. Such symmetry breaking will not always occur, and the problems encountered during the determination of the chirality of miyakosyne A1 demonstrate that the interplay between chiral guests and the nonchiral host is not yet sufficiently understood. We have therefore systematically investigated the role of chirality in the crystalline sponge method by studying the incorporation of three chiral compounds. We have selected camphene, α-pinene, and β-pinene (Figure 1) and introduced these compounds into the MOF host crystal, using either enantiopure or racemic mixtures. Camphene is a chiral hydrocarbon that crystallizes as a “plastic crystal”, meaning that molecules have (rotational) freedom to move within the crystal lattice.28,29 This means that the structure of camphene could not be resolved using “classical” X-ray diffraction methods, and therefore, it has only been investigated using other methods.30,31 Pinene is also a chiral hydrocarbon and is a liquid at room temperature. The structure of pinene has been resolved only for enantiomerically pure α-pinene using X-ray analysis. Therefore, camphene and pinene are interesting candidates for the crystalline sponge method, and we will show that their chiral form determines whether the crystalline sponge method is successful or not. Powder diffraction proved to be a crucial method to support this observation. By employing time-resolved powder X-ray diffraction, we can directly observe that only the racemic mixture of α-pinene results in ordered structures of enantiomeric pairs that can be fully resolved using single-crystal X-ray diffraction.
Figure 1.
Molecular structure of (−)-camphene (A), (+)-α-pinene (B), and (−)-β-pinene (C).
Methods and Materials
Materials were purchased from commercial sources and were used without further purification. Single crystals of the MOF host crystal were synthesized following the improved protocol described by Ramadhar et al.2 In short, 1 mL of 0.03 M ZnI2 was layered on top of 4 mL of 0.05 M tpt (2,4,6-tri(pyridin-4-yl)-1,3,5-triazine) in a glass test tube (5 mL), yielding suitable crystals after 2 days. Guest inclusion was established by exposing the crystals to a 106 M (7 g of camphene in 0.5 mL of chloroform) solution of kosher camphene (purity 90%) in chloroform or saturated camphene in a cyclohexane solution, or by exposing the MOF to pure liquid (+)-α-pinene (purity 98%) for 1 day. We used a mixture of RR-(+)-α-pinene and SS-(−)-α-pinene 1:1 v/v to study the uptake of the racemic compound in the host crystal (purity of (−)-α-pinene 97%). Note that other stereoisomers for α-pinene are impossible because of the ring structure. (−)-β-Pinene was included in the host crystal by soaking the MOF host crystal for 1 day in pure (−)-β-pinene (purity 99%). The structural isomers (+)-α-pinene and (−)-β-pinene were included by soaking the MOF host crystal for 1 day in a 1:1 v/v mixture of both compounds.
The crystalline sponge method in some cases requires only nanograms of material for successful guest inclusion and subsequent structure elucidation.1 It has since been noted that the method works best when working in neat solutions (i.e., pure liquid guest molecules).2 We studied the required concentration for successful camphene uptake and found that in the case of camphene no guest was included at concentrations of 10 M or lower, meaning that micrograms of material are required.
Fujita et al. exchanged the nitrobenzene solvent, present in the MOF, with cyclohexane, to enhance guest uptake.1 In our case chloroform was exchanged for cyclohexane to investigate whether this would lower the required camphene concentration, however, to no effect. This shows that very high concentrations are required for successful ordered camphene uptake and that both chloroform and cyclohexane exchange comparably well for camphene.
For the purpose of investigating the structures we used single-crystal X-ray diffraction (SCD). In addition we have used powder X-ray diffraction (PXRD) in order to monitor guest exchange. When a PXRD pattern of the MOF harboring a guest is simulated, diffraction peaks at low 2θ angles are predicted. The emergence of such peaks in a diffraction experiment could shed light on ordered guest inclusion and can potentially be used as a first indication of successful ordering of a guest inside the MOF, before applying SCD. Therefore, PXRD was used to study the potential ordering of both racemic and enantiomerically pure camphene and α-pinene.
X-ray Crystal Structure Determinations
Reflections were measured on a Bruker D8 Quest diffractometer with sealed tube and a Triumph monochromator (λ = 0.71073 Å) at a temperature of 150 K. The software package used for the intensity integration was Saint.32 Absorption correction and scaling were performed with SADABS.33 The structures were solved using SHELXT.34 Least-squares refinements were performed with SHELXL-201435 against F2 of all reflections. Hydrogen atoms were placed at calculated positions and were subsequently refined using a riding model. Geometry calculations and checking for higher symmetry were performed with the PLATON program.36 For all structures the SQUEEZE procedure within PLATON36 was applied for handling unordered solvent. Table 1 summarizes the most important crystal data and SQUEEZE details for the four crystal structures described in the main text.
Table 1. Summary of Crystal Data and Structure Refinementa.
| guest compound | (−)-β-pinene | rac. camphene | rac. α-pinene | (−)-β-pinene and (+)-α-pinene |
|---|---|---|---|---|
| experimental formula | C72H48I12N24Zn6, 2.709(C10H16) | C36H24I6N12Zn3, 0.824(C10H16) | C36H24I6N12Zn3, 2.481(C10H16) | C36H24I6N12Zn3, 2.819 (C10H16) |
| Fw | 3573.04 | 1718.41 | 1990.86 | 1990.86 |
| crystal system | monoclinic | monoclinic | monoclinic | monoclinic |
| space group | C2 | C2/c | C2/c | C2/c |
| a, Å | 35.587(4) | 35.416(3) | 35.972(7) | 35.62(2) |
| b, Å | 14.8874(17) | 14.8953(11) | 14.941(3) | 14.884(8) |
| c, Å | 30.854(4) | 30.600(2) | 31.398(6) | 30.831(19) |
| α/° | 90 | 90 | 90 | 90 |
| β/° | 101.678(5) | 101.875(2) | 102.06(3) | 101.63(2) |
| γ/° | 90 | 90 | 90 | 90 |
| V/Å3 | 16008(3) | 15797(2) | 16503(6) | 16011(17) |
| Z | 4 | 8 | 8 | 8 |
| guest occupancy | 1.00, 0.91, 0.80 | 0.82 | 0.84, 0.83, 0.80 | 1.00, 0.89, 0.92 |
| Dcalc/g cm–3 | 1.483 | 1.445 | 1.603 | 1.652 |
| μ/mm–1 | 3.239 | 3.279 | 3.151 | 3.248 |
| F(000) | 6768 | 6464 | 7680 | 7680 |
| GOF | 1.047 | 1.022 | 1.030 | 1.016 |
| R1, wR2 | 0.0558, 0.1340 | 0.0626, 0.1698 | 0.0531, 0.1398 | 0.0572, 0.1365 |
| R1, wR2 (all data) | 0.0876, 0.1516 | 0.0961, 0.1952 | 0.0735, 0.1569 | 0.1009, 0.1587 |
| solvent accessible volume (Squeeze) | 5147 | 5928 | 1960 | 1618 |
| electrons found in S.A.V. (Squeeze) | 1669 | 1742 | 484 | 402 |
R1 = ∑||F0| – |Fc||/∑|F0|. wR2 = {∑[w(F02 – Fc)2]/∑[w(F02)2]}1/2.
Powder diffractograms were measured on a Panalytical Empyrean diffractometer in transmission mode with fine-focus sealed tube, focusing mirror and PIXcel3D detector using Cu–K(α) radiation. The samples were measured in between two 6 μm Mylar foils. Some images were created using the CCDC Mercury software.37
Results and Discussion
First, we investigated the uptake of enantiopure (−)-β-pinene (purity 99%) into the MOF host crystal, consisting of ZnI2 and 2,4,6-tri(pyridin-4-yl)-1,3,5-triazine,1,2 to see if a loss of the inversion symmetry was observed and if the structure of the guest compound could be resolved. SCD did indeed indicate a loss of symmetry (transition from space group C2/c to C2) and revealed three (−)-β-pinene molecules in the asymmetric unit. The Flack parameter was refined to a value of 0.13(3), and this is not as close to zero as one would expect. This perhaps indicates that some inclusion of the opposite enantiomer in different twin zones has taken place (purity is 99%).
Second, we investigated the uptake of enantiopure (+)-α-pinene (purity 98%) into the MOF host crystal, to see if a loss of the inversion symmetry was again observed and if the structure of the guest compound could be resolved. SCD did not indicate a loss of symmetry and did not reveal any identifiable (+)-α-pinene molecules. Only unidentifiable small fragments of unordered solvent and/or (+)-α-pinene molecules were found inside the channels. This result is in agreement with the observation of the group of Fujita22 for α-pinene. The absence of the original chloroform solvent molecules in the structure indicated, however, that guest exchange had occurred.
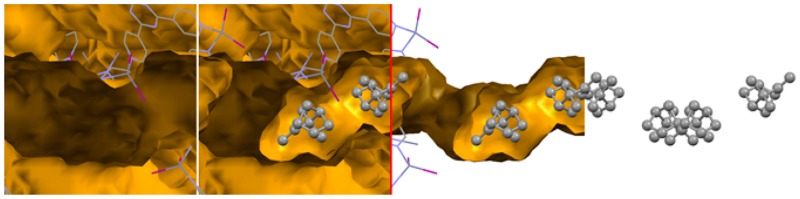
Third, we investigated the uptake of nearly enantiopure (+)-camphene into the MOF host crystal, to see if a loss of the inversion symmetry was observed and if the structure of the guest compound could be resolved. The obtained crystal structure reveals a single molecule of (+)-camphene in the asymmetric unit (Figure 2). Surprisingly, the symmetry of the system (C2/c) remained unchanged compared to the MOF host crystal. Therefore, eight camphene molecules are present inside a unit cell, four of which consist of (+)-camphene, and four of (−)-camphene. (−)-Camphene is a known impurity of commercial (+)-camphene, which in this case was only 90% pure. Therefore, the system preferred to extract and order sets of (+)-camphene and (−)-camphene molecules and keep the inversion symmetry intact, instead of only incorporating ordered (+)-camphene molecules and losing its inversion symmetry. The MOF crystals can therefore, in principle, be used to perform chiral purification. We were, however, unable to investigate this because racemic mixtures of camphene and α-pinene did not separate on chiral HPLC. The MOF is not completely filled with ordered camphene, which is more or less the case for α-pinene, and the channels also contain unordered camphene and solvent molecules which show up as diffuse electron density. Furthermore, we found that camphene could only be resolved when a concentration of 37 M or higher was used.
Figure 2.
Structure of racemic camphene in the MOF channels viewed along the crystallographic c-direction. Red and green axes are the crystallographic a- and b-direction, respectively. The host network is represented as a wireframe, while the guest is shown in a ball-and-stick fashion. The channels inside the MOF are indicated with yellow/ochre, and hydrogen atoms are omitted for clarity.
The presence of channels in all three crystallographic directions makes it possible for the (−)-camphene and (+)-camphene to easily move around and optimize their ordering inside the host channels. This explains why ordering of enantiomeric pairs is possible in this MOF host crystal, although an excess of one enantiomer is present in solution. However, one could expect that other types of MOF host crystals with a similar symmetry but with channels in only one or two directions will not give any usable results for enantiomeric pairs.
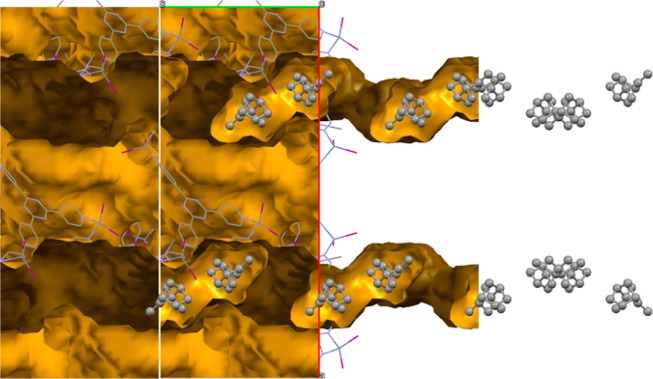
Inspired by the result for camphene, we exposed an MOF host crystal to racemic (±)-α-pinene for 24 h. The obtained crystal structure revealed three molecules in the asymmetric unit, leading to 24 molecules in the unit cell, 12 of which are (+)-α-pinene, and 12 (−)-α-pinene (Figure 3). Apparently, the system again prefers to order sets of α-pinene molecules according to the space group symmetry of the host, which includes an inversion center. We found that long exposure of the MOF to racemic α-pinene leads to more disorder in the guest structure, and eventually only diffuse electron density inside the channels and partial degradation of the host structure. Consequently, an MOF crystal that was exposed to racemic α-pinene for 22 days did not reveal any identifiable α-pinene guest molecules anymore.
Figure 3.
Structure of racemic α-pinene in the MOF channels viewed along the crystallographic c-direction. Red and green axes are the crystallographic a- and b-direction, respectively. The host network is represented as a wireframe, while the guest is shown in a ball-and-stick fashion. The channels inside the MOF are indicated with yellow/ochre, and hydrogen atoms are omitted for clarity.
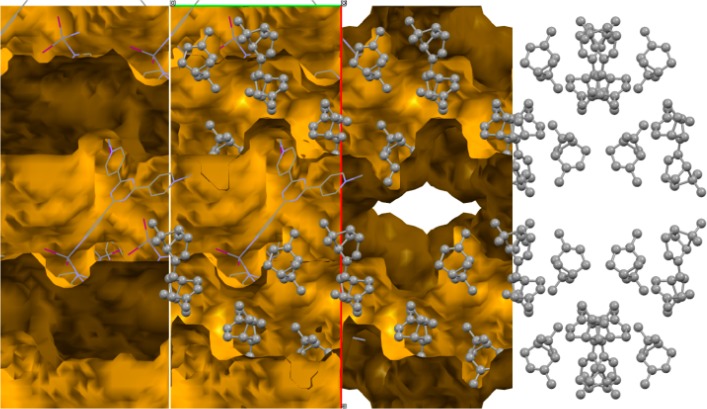
To further illustrate that the host framework has a preference for racemic mixtures, we also studied the combination of (+)-α-pinene, and (−)-β-pinene, which can almost be considered as mirror images (Figure 1). The combination is interesting because in this case the inversion symmetry of the host can almost be retained when these two isomers are present. With some difficulty the structure of pinene could indeed be established from SCD, and these structures once more showed ordering of the isomeric pairs around an inversion center, with disorder around the double bonds of the pinene structure, as one would expect. This experiment again demonstrates the selectivity of the host for racemic mixtures.
Others observed that enantiopure compounds can be ordered inside the host channels and give rise to loss of inversion symmetry, possibly aided by solvents.2,22,25−27 This is indeed the case for β-pinene but we did not observe this for camphene and α-pinene. It is not clear if ordering of enantiopure compounds or of enantiomeric pairs is most common. In any case, these examples show that resolving the chirality of a compound using the crystalline sponge method is not always straightforward. This may also explain why the structure of miyakosyne A could not be resolved straightforwardly using the crystalline sponge method, because miyakosyne A is not racemic and might not order properly due to a nonoptimal match of symmetry between guest and host system. Resolving the structure of enantiopure compounds may therefore benefit from a chiral MOF host, which does not impose a centrosymmetric ordering on guest molecules.
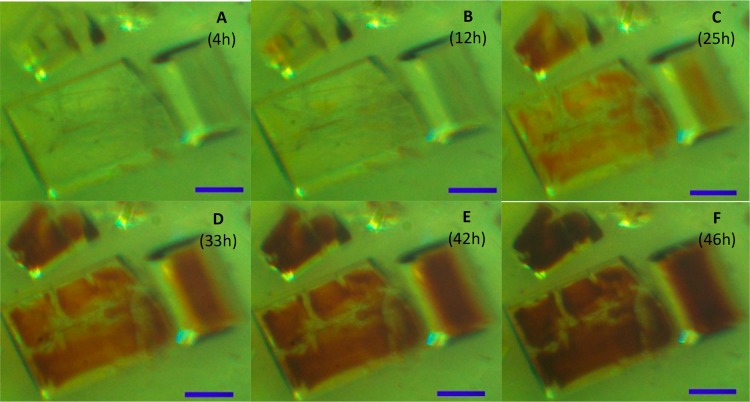
A fast method that determines whether ordered guest uptake has been established or not can save time by preventing measurement of MOF crystals only filled with solvent or unordered guest molecules giving only diffuse electron density. Previously it has been reported by Fujita et al.1 that the color change of the crystal can indicate whether guests have successfully been included, when colored guests are introduced into the MOF crystals. We observed a color change toward red in all our guest exchange experiments, while the investigated guest compounds are colorless. We also observed that resolution of the structures of guest molecules was more difficult or impossible when “older” red crystals were used. In the case of MOFs exposed to (+)-α-pinene and (−)-β-pinene, crystals changed color from the inside out (Figure 4), instead of homogeneously throughout the whole crystal. This shows that the coloring in this case is an indication for guest-induced degradation of the host framework, which is confirmed by the poorer quality of the crystal structure from diffraction experiments.
Figure 4.
MOF crystals after exposure to racemic (+)-α-pinene and (−)-β-pinene. The scale bar represents 500 μm.
Color change may indicate that guest molecules have been included inside the MOF, but does not give information regarding the ordering of guests inside the MOF host crystal. We found that PXRD patterns simulated from related MOF structures in the CSD, which include ordered guest molecules, show intensity at low 2θ angles, while the MOF host crystal containing solvent does not. If such a diffraction peak does emerge during MOF-host soaking experiments, it could help establishing the required or optimal time for guest uptake, although there may be a significant spread in optimal soaking time between different crystals, and different host–guest systems.
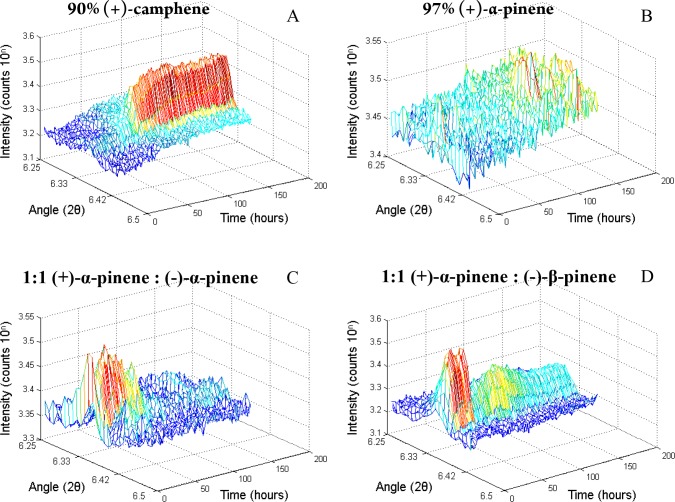
A sample of MOF crystals was continually scanned using PXRD while exposed to a camphene solution (Figure 5A). A diffraction peak clearly emerged at an angle of 2θ = 6.4° and stabilized after 3 days. This suggests that the guest uptake/ordering is completed not until this stabilization appears. A structure that was measured after 1 day, however, did not differ from the one obtained from a MOF crystal that was soaked in the same camphene solution for a period of 8 days. Therefore, it is the emergence of a PXRD peak that indicates that ordered guest uptake has sufficiently taken place to perform a single-crystal diffraction experiment.
Figure 5.
(A–D) PXRD pattern of the MOF as a function of time. The y-axis depicts the diffraction intensity (10 to the power n counts, where n is the number depicted on the axis), and the horizontal axes depict the diffraction angle (2θ), and the elapsed time (hours).
From the results of SCD, PXRD peaks are also expected in the cases of racemic α-pinene and the mixture of (+)-α-pinene and (−)-β-pinene, while no diffraction intensity is expected in the case of enantiopure (+)-α-pinene. This was confirmed with our in situ PXRD experiments (Figure 5B–D). In the case of racemic α-pinene, however, the signal dropped and stabilized to within noise level after 3 days. On the basis of these findings, a PXRD pattern should be measured already after several hours of guest introduction to establish whether ordered uptake takes place or not.
Conclusion
In summary, the MOF that was introduced by Fujita et al.1 was used to resolve enantiopure β-pinene and racemic camphene and α-pinene. For camphene and α-pinene, the host compound of Fujita shows a selectivity for enantiomeric pairs and at the same time no ordering of the enantiopure compounds. This means that the crystalline sponge method can also successfully be applied to racemic mixtures, which enlarges the scope of this method for synthetic chemists. We suggest that new MOF hosts with a chiral space group, such as the one introduced by Yaghi et al.,14 or MOF hosts containing chiral components, as recently reported by Fujita et al.,38 could simplify the resolution of chiral enantiopure compounds using the crystalline sponge method.
We showed that PXRD is a tool to investigate whether ordered guest inclusion takes place or not, and moreover, PXRD can also shed light on the kinetics of guest inclusion.
Acknowledgments
This research received funding from The Netherlands Organization for Scientific Research (NWO) in the framework of the New Chemical Innovations Fund, DSM Food Specialties, and DSM Resolve.
Accession Codes
CCDC 1549630–1549631 and 1583367–1583368 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
References
- Inokuma Y.; Yoshioka S.; Ariyoshi J.; Arai T.; Hitora Y.; Takada K.; Matsunaga S.; Rissanen K.; Fujita M. X-ray analysis on the nanogram to microgram scale using porous complexes. Nature 2013, 495 (7442), 461. 10.1038/nature11990. [DOI] [PubMed] [Google Scholar]
- Ramadhar T. R.; Zheng S. L.; Chen Y. S.; Clardy J. Analysis of rapidly synthesized guest-filled porous complexes with synchrotron radiation: practical guidelines for the crystalline sponge method. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 46–58. 10.1107/S2053273314019573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatov D. V.; Ripmeester J. A. Inclusion in microporous beta-bis(1,1,1-trifluoro-5,5-dimethyl-5-methoxyacetylacetonato)copper(II), an organic zeolite mimic. Chem. Mater. 2000, 12 (7), 1827–1839. 10.1021/cm990759d. [DOI] [Google Scholar]
- Byrn M. P.; Curtis C. J.; Hsiou Y.; Khan S. I.; Sawin P. A.; Tendick S. K.; Terzis A.; Strouse C. E. Porphyrin Sponges - Conservation of Host Structure in over 200 Porphyrin-based lattice Clathrates. J. Am. Chem. Soc. 1993, 115 (21), 9480–9497. 10.1021/ja00074a013. [DOI] [Google Scholar]
- Byrn M. P.; Curtis C. J.; Goldberg I.; Huang T.; Hsiou Y.; Khan S. I.; Sawin P. A.; Tendick S. K.; Terzis A.; Strouse C. E. Porphyrin Sponges - Programmable Lattice Clathrates. Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A 1992, 211, 135–140. 10.1080/10587259208025812. [DOI] [Google Scholar]
- Byrn M. P.; Curtis C. J.; Goldberg I.; Hsiou Y.; Khan S. I.; Sawin P. A.; Tendick S. K.; Strouse C. E. Porphyrin Sponges - Structural Systematics of the Host Lattice. J. Am. Chem. Soc. 1991, 113 (17), 6549–6557. 10.1021/ja00017a028. [DOI] [Google Scholar]
- Sanna E.; Escudero-Adan E. C.; Bauza A.; Ballester P.; Frontera A.; Rotger C.; Costa A. A crystalline sponge based on dispersive forces suitable for X-ray structure determination of included molecular guests. Chemical Science 2015, 6 (10), 5466–5472. 10.1039/C5SC01838B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biradha K.; Domasevitch K. V.; Hogg C.; Moulton B.; Power K. N.; Zaworotko M. J. Interpenetrating covalent and noncovalent nets in the crystal structures of [M(4,4′-bipyridine)2(NO3)2]·3C10H8 (M = Co, Ni). Cryst. Eng. 1999, 2 (1), 37–45. 10.1016/S1463-0184(99)00005-2. [DOI] [Google Scholar]
- Biradha K.; Domasevitch K. V.; Moulton B.; Seward C.; Zaworotko M. J. Covalent and noncovalent interpenetrating planar networks in the crystal structure of {[Ni(4,4[prime or minute]-bipyridine)2(NO3)2[middle dot]2pyrene}. Chem. Commun. 1999, (14), 1327–1328. 10.1039/a901311c. [DOI] [Google Scholar]
- Vinogradova E. V.; Muller P.; Buchwald S. L. Structural Reevaluation of the Electrophilic Hypervalent Iodine Reagent for Trifluoromethylthiolation Supported by the Crystalline Sponge Method for X-ray Analysis. Angew. Chem., Int. Ed. 2014, 53 (12), 3125–3128. 10.1002/anie.201310897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K.; Akasaka K.; Matsunaga S. Chemoenzymatic synthesis and HPLC analysis of the stereoisomers of miyakosyne A (4E,24E)-14-methyloctacosa-4,24-diene-1,27diyne-3,26-diol, a cytotoxic metabolite of a marine sponge Petrosia sp., to determine the absolute configuration of its major component as 3R,14R,26R. Tetrahedron 2014, 70 (2), 392–401. 10.1016/j.tet.2013.11.045. [DOI] [Google Scholar]
- Kawano M.; Fujita M. Direct observation of crystalline-state guest exchange in coordination networks. Coord. Chem. Rev. 2007, 251 (21–24), 2592–2605. 10.1016/j.ccr.2007.07.022. [DOI] [Google Scholar]
- Zigon N.; Hoshino M.; Yoshioka S.; Inokuma Y.; Fujita M. Where is the Oxygen? Structural Analysis of -Humulene Oxidation Products by the Crystalline Sponge Method. Angew. Chem., Int. Ed. 2015, 54 (31), 9033–9037. 10.1002/anie.201502302. [DOI] [PubMed] [Google Scholar]
- Lee S.; Kapustin E. A.; Yaghi O. M. Coordinative alignment of molecules in chiral metal-organic frameworks. Science 2016, 353 (6301), 808–811. 10.1126/science.aaf9135. [DOI] [PubMed] [Google Scholar]
- Haneda T.; Kawano M.; Kojima T.; Fujita M. Thermo-to-photo-switching of the chromic behavior of salicylideneanilines by inclusion in a porous coordination network. Angew. Chem., Int. Ed. 2007, 46 (35), 6643–6645. 10.1002/anie.200700999. [DOI] [PubMed] [Google Scholar]
- Millange F.; Serre C.; Guillou N.; Ferey G.; Walton R. I. Structural effects of solvents on the breathing of metal-organic frameworks: An in situ diffraction study. Angew. Chem., Int. Ed. 2008, 47 (22), 4100–4105. 10.1002/anie.200705607. [DOI] [PubMed] [Google Scholar]
- Meilikhov M.; Yusenko K.; Fischer R. A. The adsorbate structure of ferrocene inside Al(OH)(bdc) (x) (MIL-53): a powder X-ray diffraction study. Dalton Transactions 2009, (4), 600–602. 10.1039/B820882B. [DOI] [PubMed] [Google Scholar]
- Sapchenko S. A.; Samsonenko D. G.; Dybtsev D. N.; Melgunov M. S.; Fedin V. P. Microporous sensor: gas sorption, guest exchange and guest-dependant luminescence of metal-organic framework. Dalton Transactions 2011, 40 (10), 2196–2203. 10.1039/C0DT00999G. [DOI] [PubMed] [Google Scholar]
- Wang X. F.; Lv Y.; Su Z.; Okamura T.; Wu G.; Sun W. Y.; Ueyama N. Anion and additive effects on the structure of Mercury(II) halides complexes with tripodal ligand. Z. Anorg. Allg. Chem. 2007, 633 (15), 2695–2700. 10.1002/zaac.200700220. [DOI] [Google Scholar]
- Nozaki T.; Kosaka W.; Miyasaka H. Honeycomb frameworks with a very large mesh of 39 [times] 29 A diameters stabilized by [small pi]-stacked coronene molecules. CrystEngComm 2012, 14 (17), 5398–5401. 10.1039/c2ce25806d. [DOI] [Google Scholar]
- Piñeiro-López L.; Arcís-Castillo Z.; Muñoz M. C.; Real J. A. Clathration of Five-Membered Aromatic Rings in the Bimetallic Spin Crossover Metal–Organic Framework [Fe(TPT)2/3{MI(CN)2}2]·G (MI = Ag, Au). Cryst. Growth Des. 2014, 14 (12), 6311–6319. 10.1021/cg5010616. [DOI] [Google Scholar]
- Zigon N.; Kikuchi T.; Ariyoshi J.; Inokuma Y.; Fujita M. Structural Elucidation of Trace Amounts of Volatile Compounds Using the Crystalline Sponge Method. Chem. - Asian J. 2017, 12 (10), 1057–1061. 10.1002/asia.201700515. [DOI] [PubMed] [Google Scholar]
- Cuenca A. B.; Zigon N.; Duplan V.; Hoshino M.; Fujita M.; Fernandez E. Undeniable Confirmation of the syn-Addition Mechanism for Metal-Free Diboration by Using the Crystalline Sponge Method. Chem. - Eur. J. 2016, 22 (14), 4723–4726. 10.1002/chem.201600392. [DOI] [PubMed] [Google Scholar]
- Duplan V.; Hoshino M.; Li W.; Honda T.; Fujita M. InSitu Observation of Thiol Michael Addition to a Reversible Covalent Drug in a Crystalline Sponge. Angew. Chem., Int. Ed. 2016, 55 (16), 4919–4923. 10.1002/anie.201509801. [DOI] [PubMed] [Google Scholar]
- Urban S.; Brkljaca R.; Hoshino M.; Lee S.; Fujita M. Determination of the Absolute Configuration of the Pseudo-Symmetric Natural Product Elatenyne by the Crystalline Sponge Method. Angew. Chem., Int. Ed. 2016, 55 (8), 2678–2682. 10.1002/anie.201509761. [DOI] [PubMed] [Google Scholar]
- Matsuda Y.; Mitsuhashi T.; Lee S.; Hoshino M.; Mori T.; Okada M.; Zhang H. P.; Hayashi F.; Fujita M.; Abe I. Astellifadiene: Structure Determination by NMR Spectroscopy and Crystalline Sponge Method, and Elucidation of its Biosynthesis. Angew. Chem., Int. Ed. 2016, 55 (19), 5785–5788. 10.1002/anie.201601448. [DOI] [PubMed] [Google Scholar]
- Inokuma Y.; Ukegawa T.; Hoshino M.; Fujita M. Structure determination of microbial metabolites by the crystalline sponge method. Chemical Science 2016, 7 (6), 3910–3913. 10.1039/C6SC00594B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning W. J. Crystallographic studies of plastic crystals. J. Phys. Chem. Solids 1961, 18 (1), 21–27. 10.1016/0022-3697(61)90079-8. [DOI] [Google Scholar]
- Green J. R.; Wheeler D. R. X-ray Investigation of Some Plastic Crystals I. Structure and Rotation: Cyclopentanol and dl-Camphene. Mol. Cryst. 1969, 6 (1), 1–11. 10.1080/15421406908082949. [DOI] [Google Scholar]
- Neeman E. M.; Drean P.; Huet T. R. The structure and molecular parameters of camphene determined by Fourier transform microwave spectroscopy and quantum chemical calculations. J. Mol. Spectrosc. 2016, 322, 50–54. 10.1016/j.jms.2016.03.012. [DOI] [Google Scholar]
- Gusevskaya E. V.; da Silva M. J. Elucidation of the stereochemistry of diterpene derivatives obtained by palladium catalyzed oxidative coupling-oxidation of camphene. J. Braz. Chem. Soc. 2003, 14 (1), 83–89. 10.1590/S0103-50532003000100014. [DOI] [Google Scholar]
- SAINT-Plus; Bruker AXS Inc.: Madison, Wisconsin, USA, 2001.
- Sheldrick G. M.SADABS and TWINABS: Area-Detector Absorption Correction; Universität Göttingen: Germany, 1999. [Google Scholar]
- Sheldrick G. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71 (1), 3–8. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71 (1), 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spek A. L. Structure validation in chemical crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2009, 65, 148–155. 10.1107/S090744490804362X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae C. F.; Bruno I. J.; Chisholm J. A.; Edgington P. R.; McCabe P.; Pidcock E.; Rodriguez-Monge L.; Taylor R.; van de Streek J.; Wood P. A. Mercury CSD 2.0 - new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. 10.1107/S0021889807067908. [DOI] [Google Scholar]
- Yan K. K.; Dubey R.; Arai T.; Inokuma Y.; Fujita M. Chiral Crystalline Sponges for the Absolute Structure Determination of Chiral Guests. J. Am. Chem. Soc. 2017, 139 (33), 11341–11344. 10.1021/jacs.7b06607. [DOI] [PubMed] [Google Scholar]