Abstract
Background:
Intervertebral disc degeneration (IDD) is a major cause of disc protrusion, likely to be associated with decrease of water content. This research aimed to evaluate IDD by diffusion-weighted imaging (DWI) with a 7.0 Tesla (T) magnetic resonance imaging (MRI) machine.
Methods:
A total of 24 healthy Sprague-Dawley rats were randomly selected and divided into four groups (A, B, C, and D), each consisting of 3 male and 3 female rats (28, 42, 56, and 70 days old, respectively). All the rats were imaged with a 7.0T MRI, producing T2WI, T1WI, and functional DWI sequences. Data were collected and apparent diffusion coefficient (ADC) charts were constructed. Nucleus pulposus and annulus fibrosus regions were identified, several regions of interest were chosen, and their ADC values were obtained. After imaging, rats were sacrificed and their intervertebral discs (L1–L6) were dissected, yielding a total of 144 discs. Protein was extracted for the purpose of Western blotting. Comparison among multiple samples used one-way analysis of variance and least significant difference methods.
Results:
7.0T MRI revealed evident decrease in signal intensity within intervertebral discs of Sprague-Dawley rats with age. Intervertebral disc ADC values significantly decreased from Group A (0.00154 ± 0.00008) to Group D (0.00107 ± 0.00007; P < 0.01); nucleus pulposus ADC values significantly decreased from Group A (0.00164 ± 0.00005) to Group D (0.00140 ± 0.00007; P < 0.01) and annulus fibrosus ADC values significantly decreased from Group A (0.00129 ± 0.00014) to Group D (0.00082 ± 0.00012; P < 0.01). Meanwhile, it also revealed evident decrease from high spinal level to low spinal level: nucleus pulposus ADC values in Group A significantly decreased from L1/L2 (0.00163 ± 0.00006) to L6/S1 (0.00139 ± 0.00004; P < 0.01). While annulus fibrosus ADC values did not differ significantly between levels in Group A (P > 0.05). Western blotting showed that aggrecan content of intervertebral discs decreased from Group A (1.88 ± 0.16) to Group D (0.17 ± 0.04) with age (P < 0.01); Type II collagen content of intervertebral discs decreased from Group A (2.22 ± 0.04) to Group D (0.20 ± 0.01) with age (P < 0.01). No significant differences in aggrecan and Type II collagen content of L1–L6 intervertebral discs in Group A were noted (P > 0.05). Mean ADC values of different intervertebral regions were positively correlated with aggrecan and Type II collagen content (aggrecan: r = 0.631, P < 0.01; Type II collagen: r = 0.680, P < 0.01).
Conclusion:
7.0T MRI-DWI could be applied to effectively diagnose and research early IDD in tiny variations.
Keywords: Apparent Diffusion Coefficient, Diffusion-weighted Imaging, Intervertebral Disc Degeneration, Magnetic Resonance Imaging
INTRODUCTION
It is widely accepted that magnetic resonance imaging (MRI) has thus become the dominant imaging modality for the evaluation of intervertebral disc degeneration (IDD).[1,2] Disc matrix which consists primarily of aggrecan and Type II collagen is the primary component of the intervertebral disc. Previous studies have suggested that the pathologic process of IDD is associated with loss of aggrecan and Type II collagen. Aggrecan is highly hydrophilic and maintains water retention, generating a swelling pressure sufficient enough to keep the body stable.[3] Such unique characteristics of this tissue provide a base for effective diagnosis of early IDD.
Although conventional MRI is frequently used to evaluate patients for IDD, imaging is often insufficient from both qualitative and quantitative perspectives.[4] Functional MRI (fMRI) has emerged as a powerful imaging modality in the recent years, and diffusion-weighted imaging (DWI) can be applied to analyze the different characteristics of tissues. DWI can noninvasively and accurately reveal detailed pathological, physiological, and even biochemical changes at the molecular level in a wide variety of regions.[5] The application of fMRI in the diagnosis of brain diseases provides a solid foundation for efficient and accurate future evaluation of IDD. This study explored the application of 7.0T MRI-DWI in the evaluation of IDD.
METHODS
Animals
In reference to comparisons between rat and human life spans, a total of 24 healthy Sprague-Dawley rats were randomly selected. These rats were subsequently randomly divided into four groups (A, B, C, and D), each consisting of 3 male and 3 female rats (28, 42, 56, and 70 days old, respectively). Intervertebral disc samples (L1–L6) were dissected from each rat, yielding a total of 144 discs. The Animal Care and Use Committee of the Affiliated Hospital of Qingdao University (Shandong, China) approved all animal experimental protocols, according to the National Institute of Health Guide.
Scan method and parameters
Four groups of Sprague-Dawley rats were anesthetized with 1.5‰ isoflurane for 5 min and both conventional MRI and DWI were used to continuously monitor their L1–L6 intervertebral discs. A 7.0T MRI Bruker PharmaScan® instrument (Bruker® Corporation, Germany) was used, and sequences for single-short spin echo planar images were applied for imaging transverse sections of lumbar intervertebral discs. Thirty directions of diffusion-sensitive gradients were applied. The imaging parameters were repetition time (TR) = 5000 ms, echo time (TE) = 104 ms, slice thickness = 5 mm, and diffusion-weighted coefficients (b value) were 0 and 670 s/mm2. The area of observation was utilized to calculate apparent diffusion coefficients (ADCs) or detection indices of DWI. With the aid of the DTI Studio system, the equatorial portion of intervertebral discs was selected for diffusion imaging. This allowed for comprehensive data collection, while IDD imaging values allowed for accurate mathematical analysis of varying degrees of natural disc degeneration by DWI. Nucleus pulposus ADC values were recorded from the 12 points of the center area, and the mean was calculated. Annulus fibrosis ADC values were recorded from the 16 points of the outer area, and the mean was calculated. Intervertebral disc ADC values from the 28 points were calculated, and the mean was calculated [Figure 1].
Figure 1.
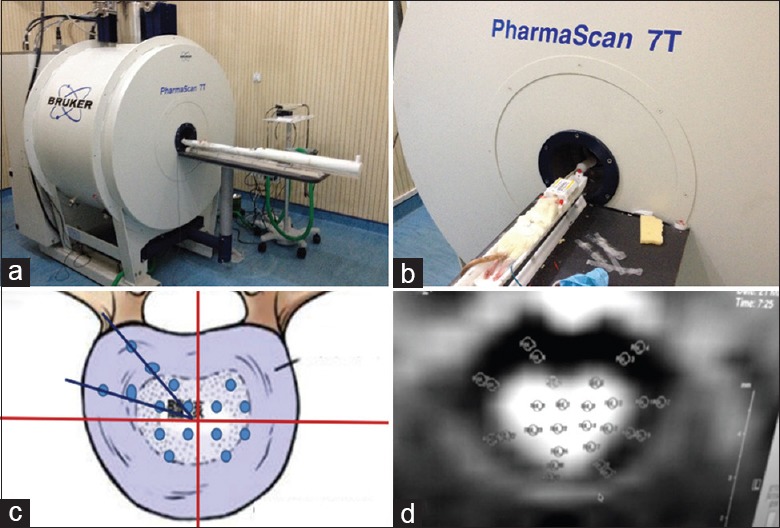
(a) 7.0T Magnetic resonance imaging and (b) body position in the magnetic resonance imaging scanning. Twenty-eight images were obtained of each imaged layer. (c) The disc (including nucleus pulposus and annulus fibrosis) was divided into four quadrants of equal area. (d) Due to uncertainty in differences regarding the distribution of fractional anisotropy values, regions of interests were manually determined from the outer annulus fibrosis, inner annulus fibrosis, and nucleus pulposus areas of each quadrant for the purpose of statistical analysis and image reconstruction.
Molecular biological detection of intervertebral disc tissue
After imaging, rats were sacrificed and intervertebral discs (L1–L6) were isolated. A total of 144 intervertebral disc samples (complete intervertebral discs from levels L1–L6) were obtained from four groups (28, 42, 56, and 70 days old). Protein was extracted for the purpose of Western blotting, and aggrecan and Type II collagen content of each intervertebral disc was determined. β-actin was applied as the internal control. Ratio of aggrecan divided by β-actin was recorded, and the ratio of Type II collagen divided by β-actin was recorded. These two ratios were calculated as the values of the corresponding intervertebral disc.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Results were analyzed using SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA) with the homogeneity test of variance and normality test was applied for data analysis. Qualified means of multiple samples were compared using one-way analysis of variance (ANOVA), while the least significant difference (LSD) pairwise comparison methods were used to compare results. A P < 0.05 was considered statistically significant.
RESULTS
Results of diffusion-weighted imaging
As shown in Table 1, we found that intervertebral disc ADC values among different age groups (Groups A, B, C, and D) decreased with age. Total ADC values of L1 intervertebral discs in Groups A (0.00154 ± 0.00008), B (0.00130 ± 0.00005), C (0.00122 ± 0.00007), and D (0.00107 ± 0.00007) significantly decreased with age (P < 0.01); nucleus pulposus ADC values of L1 intervertebral discs in Groups A (0.00164 ± 0.00005), B (0.00156 ± 0.00007), C (0.00147 ± 0.00008), and D (0.00140 ± 0.00007) significantly decreased with age (P < 0.01); annulus fibrosis ADC values of L1 intervertebral discs in Groups A (0.00129 ± 0.00014), B (0.00110 ± 0.00011), C (0.00095 ± 0.00009), and D (0.00082 ± 0.00012) significantly decreased with age (P < 0.01). Moreover, a comparison of nucleus pulposus and annulus fibrosis ADC values of different spinal levels in Group A was performed [Table 2]. Nucleus pulposus and annulus fibrosis (L1–L6) in Group A were measured, and the statistical analysis of obtained ADC values suggested that nucleus pulposus ADC values in Group A from level L1/L2 (0.00163 ± 0.00006), L2/L3 (0.00154 ± 0.00006), L3/L4 (0.00155 ± 0.00005), L4/L5 (0.00152 ± 0.00004), L5/L6 (0.0015 ± 0.00004), and L6/S1 (0.00139 ± 0.00004) significantly decreased (P < 0.01). However, there were no statistically significant differences in annulus fibrosis ADC values of different spinal levels in Group A (P > 0.05).
Table 1.
Intervertebral disc ADC values of rats from A, B, C, and D groups
| Position | Group A (‰) | Group B (‰) | Group C (‰) | Group D (‰) | F | P |
|---|---|---|---|---|---|---|
| ID | 1.54 ± 0.08 | 1.30 ± 0.05 | 1.22 ± 0.07 | 1.07 ± 0.07 | 71.67 | <0.01 |
| NP | 1.64 ± 0.05 | 1.56 ± 0.07 | 1.47 ± 0.08 | 1.40 ± 0.07 | 20.02 | <0.01 |
| AF | 1.29 ± 0.14 | 1.10 ± 0.11 | 0.95 ± 0.09 | 0.82 ± 0.12 | 27.01 | <0.01 |
Data were shown as mean ± SD. Group A: 28 days old; Group B: 42 days old; Group C: 56 days old; Group D: 70 days old; ID: Intervertebral disc; NP: Nucleus pulposus; AF: Annulus fibrosis; SD: Standard deviation; ADC: Apparent diffusion coefficient. n=6 in each group.
Table 2.
Nucleus pulposus and annulus fibrosis ADC values of different levels in Group A (n=6)
| Position | L1/L2 (‰) | L2/L3 (‰) | L3/L4 (‰) | L4/L5 (‰) | L5/L6 (‰) | L6/S1 (‰) | F | P |
|---|---|---|---|---|---|---|---|---|
| NP | 1.63 ± 0.06 | 1.54 ± 0.06 | 1.55 ± 0.05 | 1.52 ± 0.04 | 1.50 ± 0.04 | 1.39 ± 0.04 | 14.99 | <0.01 |
| AF | 1.11 ± 0.18 | 1.14 ± 0.19 | 1.36 ± 0.14 | 1.21 ± 0.13 | 1.14 ± 0.12 | 1.31 ± 0.13 | 3.17 | >0.05 |
Data were shown as mean ± SD. NP: Nucleus pulposus; AF: Annulus fibrosis; SD: Standard deviation; ADC: Apparent diffusion coefficient.
Western blotting
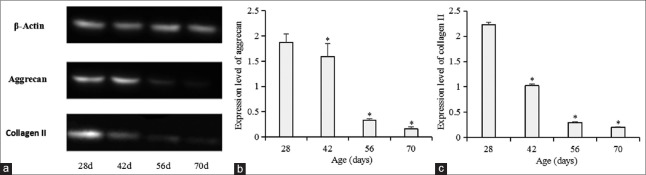
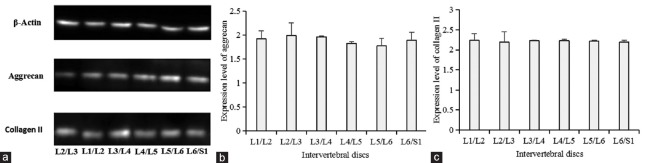
Intervertebral discs were isolated and Western blotting was applied to detect protein levels in intervertebral discs [Table 3 and Figure 2]. Protein expression of L1 intervertebral discs among different age groups showed that aggrecan content of intervertebral discs in Groups A (1.88 ± 0.16), B (1.59 ± 0.26), C (0.33 ± 0.02), and D (0.17 ± 0.04) significantly decreased with age (P < 0.01); collagen II content of intervertebral discs in Groups A (2.22 ± 0.04), B (1.02 ± 0.03), C (0.29 ± 0.02), and D (0.20 ± 0.01) significantly decreased with age (P < 0.01). L1-L6 intervertebral discs of the rats from Group A were isolated and prepared for Western blotting analysis to detect protein levels in discs. We compared aggrecan and collagen II content of different intervertebral disc levels in Group A [Table 4 and Figure 3]. However, no significant differences in aggrecan and collagen II content of L1–L6 intervertebral discs in Group A were noted (P > 0.05).
Table 3.
Protein content of L1 intervertebral discs among different age groups
| Target protein | Group A | Group B | Group C | Group D | F | P |
|---|---|---|---|---|---|---|
| Aggrecan | 1.88 ± 0.16 | 1.59 ± 0.26 | 0.33 ± 0.02 | 0.17 ± 0.04 | 188.77 | <0.01 |
| Type II collagen | 2.22 ± 0.04 | 1.02 ± 0.03 | 0.29 ± 0.02 | 0.20 ± 0.01 | 6240.78 | <0.01 |
Data were shown as mean ± SD. Group A: 28 days old; Group B: 42 days old; Group C: 56 days old; Group D: 70 days old; SD: Standard deviation. n=6 in each group.
Figure 2.
(a) Protein expression of L1 intervertebral discs between different age groups. (b) Analysis of aggrecan expression of L1 intervertebral discs among 28-, 42-, 56-, and 70-day groups (*P < 0.05 compared with the 28 days’ group). (c) Analysis of collagen II expression of L1 intervertebral discs among 28-, 42-, 56-, and 70-day-old groups (*P < 0.01 compared with 28-day-old group).
Table 4.
Protein content of L1–L6 intervertebral discs in Group A (n=6)
| Target protein | L1/L2 | L2/L3 | L3/L4 | L4/L5 | L5/L6 | L6/S1 | F | P |
|---|---|---|---|---|---|---|---|---|
| Aggrecan | 1.92 ± 0.16 | 1.99 ± 0.17 | 1.96 ± 0.26 | 1.83 ± 0.17 | 1.77 ± 0.09 | 1.89 ± 0.19 | 1.26 | >0.05 |
| Type II collagen | 2.24 ± 0.03 | 2.19 ± 0.05 | 2.23 ± 0.05 | 2.22 ± 0.03 | 2.21 ± 0.03 | 2.20 ± 0.02 | 1.49 | >0.05 |
Data were shown as mean ± SD. SD: Standard deviation.
Figure 3.
(a) Protein expression of different intervertebral discs in Group A. (b) Analysis of aggrecan expression from L1/L2 to L6/S1 level intervertebral discs in Group A (P > 0.05). (c) Analysis of collagen II expression from L1/L2 to L6/S1 level intervertebral discs in Group A (P > 0.05).
Correlation between apparent diffusion coefficient values and protein expression levels
Correlation analysis was performed on both level L1 intervertebral disc ADC values obtained from imaging and content of aggrecan and Type II collagen obtained from biochemical tests. Results revealed a significantly positive correlation (aggrecan: r = 0.631, P < 0.01; Type II collagen: r = 0.680, P < 0.01).
DISCUSSION
Multiple factors including cellular processes, extracellular matrix physiology, cytokines, biomechanics, and genetics influence IDD.[6,7,8,9] Decreased matrix content as a major etiology, however, has been the focus of the majority of recent studies.
In vitro and in vivo studies have suggested that aggrecan and Type II collagen serve important roles in the pathogenesis of IDD.[10,11] Proteoglycan aggregates in adult human nucleus pulposus are mainly distributed within the extracellular matrix, while those of the annulus fibrosis are mainly distributed between collagen fibers. Aggrecan content increases progressively from the outer annulus fibrosis toward the center of the nucleus pulposus.[12] Since aggrecan maintains adequate hydration, the nucleus pulposus has a significantly higher water content than the annulus fibrosis does. In addition, the nucleus pulposus is also more elastic.
Wu et al.[13] found proteoglycan aggregates concentration to decrease as age increased. As a result, the intervertebral disc becomes more brittle and loses stability. Type II collagen is the most widely distributed collagen in normal human intervertebral discs, which shows a progressive increase from the periphery toward the center of the nucleus pulposus.[14] Aggrecan, on the other hand, is integrated into a network frame with collagen. These characteristics not only can be applied to imaging analysis on the basis of molecular motion, but also provide a foundation for studying biochemical components of intervertebral discs in great detail. The influences of proteoglycans on IDD pathogenesis have not been well quantified to date, and our study provides a potentially useful method for evaluating pathophysiological interactions within the rat intervertebral discs via 7.0T MRI.
The Pfirrmann grading system, closely related to signal strengths of nucleus pulposus and annulus fibrosis, is widely used in the clinical evaluation of IDD.[15] However, it is neither accurate nor particularly applicable for diagnosing early disc degeneration, in large part due to subjectivity of the examiner, weak classification effects on aged intervertebral discs, and insufficient capability for accurately assessing the degree of degeneration. A new IDD score system is required for this purpose, along with the ability to accurately detail the degree of disc degeneration.
It is therefore of vital importance to explore higher level imaging techniques to study more subtle changes in IDD. Among the various types of MRIs, DWI is mainly used for detecting differences in water content of tissues. In intervertebral discs, water molecules are mainly distributed within the extracellular matrix and DWI can detect diffusion movement and distribution of water molecules. When there is degeneration of intervertebral discs, the movement of free water molecules in tissues becomes limited and their distribution gets altered. DWI can display changes in molecular diffusion of water in tissues and their microstructures.
Previous studies have reported DWI to be highly sensitive in displaying early functional changes in tissues. When biochemical compositions of tissues are altered, the diffusion of water molecules is affected and disc degeneration can be detected rapidly by analyzing changes in ADC values.[16,17] However, as 1.5T and 3.0T MRI are most widely used, these magnetic strengths are not precise or detailed enough for efficient tissue imaging.[18,19] High-intensity MRI, on the other hand, can be useful.
DWI imaging is based on molecular flow effects where its signals are derived from free water. DWI, analyzing the diffusion coefficient of fluid in vivo, is the noninvasive way to detect both intra- and extracellular molecular diffusion of water in living organisms.[20,21] DWI is used to detect molecular changes of water surface tension under gradient changes of field intensity. Compared with spectrum technology, this method does not depend on the concentration of compounds of interest and as such is more sensitive in the diagnosis of early IDD.[22] As the intervertebral disc is among the largest avascular tissues in the body, quantitative diffusion techniques can achieve effective real-time monitoring of intervertebral disc composition. A previous study reported water content to decrease with the degenerative process of the intervertebral disc.[23]
To our knowledge, few studies have reported investigating the use of 7.0T MRI as a diagnostic approach or in evaluating the course of IDD in vivo. We established a degeneration model using intervertebral discs of SD rats and studied their natural degeneration with the aid of 7.0T MRI. Our results indicate that the ADC values of nucleus pulposus and annulus fibrosis decrease with age, these findings are in agreement with those of Kealey et al.,[24] who reported a decrease of 9% in ADC values as age increased. Lower ADC values were found in the caudal vertebra. Antoniou et al.[25] and Maasumi et al.[26] reported that ADC values also decreased as the severity of IDD progressed. Our experiments utilized SD rats and we found gradually decreasing ADC values of nucleus pulposus with increasing degeneration of spinal segments. The volume of annulus fibrosis was not obviously changed. Similarly, biochemical test results revealed no significant differences between segments in intervertebral disc protein expression, which possibly was associated with the volume of intervertebral disc in the animals.
In addition, fMRI revealed ADC values of corresponding areas in nuclei pulposi and annuli fibrosi of the same segment of intervertebral discs in four groups of SD rats to also decrease as the rats aged. This suggests major changes in water content within intervertebral discs, consistent with models of their natural degeneration. In addition, biochemical tests indicated that aggrecan and Type II collagen content in discs decline as rats aged. A gradual decline in water content was also apparent.[27] Degeneration of the nuclei pulposi and annuli fibrosi was therefore quantitatively assessed by changes in ADC values. Our study further confirmed the feasibility of DWI in assessing relevant changes in the water content of intervertebral discs for the purposes of IDD assessment. Our experiment also found that ADC values correlated well with changes in aggrecan and Type II collagen in the extracellular matrix. Data obtained from DWI assessments could thus indirectly reflect the water content of discs and assess the degree of IDD via changes in ADC values.
7.0T FMR-DWI is a sensitive, safe, and noninvasive imaging modality not involving radiation. It can not only accurately detect early IDD, but also displays biological changes of molecules in all intervertebral discs imaged; in addition, it can accurately detect the range and degree of degeneration within the same individual as reference. These characteristics make FMR-DWI rather suitable for studying intervertebral discs. Our study applied MRI at 3.0T to quantitatively assess the degeneration of human lumbar intervertebral discs.[28] MRI, however, is rarely reported to be used in the quantitative evaluation of early IDD. Utilizing SD rats as models, this study has explored MRI to be certainly feasible in diagnosing human IDD.
This study also aimed to evaluate the associations of water content, aggrecan, Type II collagen, and IDD within the lumbar spines of rats. We also aimed to improve the detection rate for diagnosing IDD. However, there is no imaging technology that can obtain a unified understanding of degeneration of the entire spinal column, or any method of data analysis that can satisfactorily analyze and explain all the obtained information simultaneously. High-resolution nuclear MRI may accurately describe the physical morphology of nucleus pulposus and annulus fibrosis and allow signal intensity data of images to be applied toward characterization of the intervertebral disc.
Furthermore, changes of water molecule diffusion in intervertebral discs are regarded as a sign of obvious early IDD, effectively detected by MRI-DWI shortly after early degeneration of the intervertebral disc begins. This study reported ADC values of DWI to be the important indicator in assessing the strength and capacity of water molecule diffusion in degenerating intervertebral discs. Signs of IDD could be detected early by studying the quantitative relations between obtained data; therefore, interventional therapy could be given to decrease degeneration in a timely manner.
This study provides a foundation for the establishment of animal models for noninvasive, quantitative IDD analysis. Clinically, application of DWI not only makes up for the shortcomings of conventional MRI in effectively imaging IDD, but also provides a novel way of approaching lower back pathology with fMRI techniques.
However, there are still some limitations in the clinical application of fMRI. First, high magnetic field cannot eliminate the interference signals in other tissues completely. The signal obtained in examination of the intervertebral disc is often mixed with many interference signals of visceral and abdominal wall tissue in front of intervertebral disc that requires manual analysis and reduces the reliability of numerical value. Second, clinical application of high-field magnetic resonance has not been clearly confirmed. There is a lack of human clinical studies on side effects of high-intensity magnetic field, and even few reports on the development of matching bioengineering equipment. Although the application of intervertebral disc in clinic is limited by many factors, the prospect of fMRI is still promising.
Financial support and sponsorship
This study was supported by a grant of the National Natural Science Foundation of China (No. 81371998).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31:2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 2.Nakashima H, Yukawa Y, Suda K, Yamagata M, Ueta T, Kato F, et al. Cervical disc protrusion correlates with the severity of cervical disc degeneration: A cross-sectional study of 1211 relatively healthy volunteers. Spine (Phila Pa 1976) 2015;40:E774–9. doi: 10.1097/BRS.0000000000000953. doi: 10.1097/BRS.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 3.Sivan SS, Roberts S, Urban JP, Menage J, Bramhill J, Campbell D, et al. Injectable hydrogels with high fixed charge density and swelling pressure for nucleus pulposus repair: Biomimetic glycosaminoglycan analogues. Acta Biomater. 2014;10:1124–33. doi: 10.1016/j.actbio.2013.11.010. doi: 10.1016/j.actbio.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Antoniou J, Epure LM, Michalek AJ, Grant MP, Iatridis JC, Mwale F, et al. Analysis of quantitative magnetic resonance imaging and biomechanical parameters on human discs with different grades of degeneration. J Magn Reson Imaging. 2013;38:1402–14. doi: 10.1002/jmri.24120. doi: 10.1002/jmri.24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SG, Ogawa S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2012;32:1188–206. doi: 10.1038/jcbfm.2012.23. doi: 10.1038/jcbfm.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh CD, Im HJ, Suh J, Chee A, An H, Chen D, et al. Rho-associated kinase inhibitor immortalizes rat nucleus pulposus and annulus fibrosus cells: Establishment of intervertebral disc cell lines with novel approaches. Spine (Phila Pa 1976) 2016;41:E255–61. doi: 10.1097/BRS.0000000000001235. doi: 10.1097/BRS.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X, Jing L, Richardson WJ, Isaacs RE, Fitch RD, Brown CR, et al. Identifying molecular phenotype of nucleus pulposus cells in human intervertebral disc with aging and degeneration. J Orthop Res. 2016;34:1316–26. doi: 10.1002/jor.23244. doi: 10.1002/jor.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Séguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 2005;30:1940–8. doi: 10.1097/01.brs.0000176188.40263.f9. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- 9.Marfia G, Campanella R, Navone SE, Zucca I, Scotti A, Figini M, et al. Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: A preliminary study on biglycan-deficient murine model of chronic disc degeneration. Arthritis Res Ther. 2014;16:457. doi: 10.1186/s13075-014-0457-5. doi: 10.1186/s13075-014-0457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruber HE, Ingram J, Leslie K, Hanley EN., Jr Gene expression of types I, II, and VI collagen, aggrecan, and chondroitin-6-sulfotransferase in the human annulus: In situ hybridization findings. Spine J. 2008;8:810–7. doi: 10.1016/j.spinee.2007.07.387. doi: 10.1016/j.spinee.2007.07.387. [DOI] [PubMed] [Google Scholar]
- 11.Rutges JP, Kummer JA, Oner FC, Verbout AJ, Castelein RJ, Roestenburg HJ, et al. Increased MMP-2 activity during intervertebral disc degeneration is correlated to MMP-14 levels. J Pathol. 2008;214:523–30. doi: 10.1002/path.2317. doi: 10.1002/path.2317. [DOI] [PubMed] [Google Scholar]
- 12.Roberts S, Caterson B, Evans H, Eisenstein SM. Proteoglycan components of the intervertebral disc and cartilage endplate: An immunolocalization study of animal and human tissues. Histochem J. 1994;26:402–11. doi: 10.1007/BF00160052. doi: 10.1007/BF00160052. [DOI] [PubMed] [Google Scholar]
- 13.Wu B, Meng C, Wang H, Jia C, Zhao Y. Changes of proteoglycan and collagen II of the adjacent intervertebral disc in the cervical instability models. Biomed Pharmacother. 2016;84:754–8. doi: 10.1016/j.biopha.2016.09.077. doi: 10.1016/j.biopha.2016.09.077. [DOI] [PubMed] [Google Scholar]
- 14.Kozaci LD, Guner A, Oktay G, Guner G. Alterations in biochemical components of extracellular matrix in intervertebral disc herniation: Role of MMP-2 and TIMP-2 in type II collagen loss. Cell Biochem Funct. 2006;24:431–6. doi: 10.1002/cbf.1250. doi: 10.1002/cbf.1250. [DOI] [PubMed] [Google Scholar]
- 15.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–8. doi: 10.1097/00007632-200109010-00011. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 16.Eguchi Y, Ohtori S, Yamashita M, Yamauchi K, Suzuki M, Orita S, et al. Diffusion-weighted magnetic resonance imaging of symptomatic nerve root of patients with lumbar disk herniation. Neuroradiology. 2011;53:633–41. doi: 10.1007/s00234-010-0801-7. doi: 10.1007/s00234-010-0801-7. [DOI] [PubMed] [Google Scholar]
- 17.Yu HJ, Bahri S, Gardner V, Muftuler LT. In vivo quantification of lumbar disc degeneration: Assessment of ADC value using a degenerative scoring system based on Pfirrmann framework. Eur Spine J. 2015;24:2442–8. doi: 10.1007/s00586-014-3721-0. doi: 10.1007/s00586-014-3721-0. [DOI] [PubMed] [Google Scholar]
- 18.Shi L, Yang AC, Meng DW, Li SW, Liu HG, Li JJ, et al. Pathological alterations and stress responses near DBS electrodes after MRI scans at 7.0T, 3.0T and 1.5T: An in vivo comparative study. PLoS One. 2014;9:e101624. doi: 10.1371/journal.pone.0101624. doi: 10.1371/journal.pone.0101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordmeyer-Massner JA, Wyss M, Andreisek G, Pruessmann KP, Hodler J. In vitro and in vivo comparison of wrist MR imaging at 3.0 and 7.0 tesla using a gradient echo sequence and identical eight-channel coil array designs. J Magn Reson Imaging. 2011;33:661–7. doi: 10.1002/jmri.22419. doi: 10.1002/jmri.22419. [DOI] [PubMed] [Google Scholar]
- 20.Chilla GS, Tan CH, Xu C, Poh CL. Diffusion weighted magnetic resonance imaging and its recent trend-a survey. Quant Imaging Med Surg. 2015;5:407–22. doi: 10.3978/j.issn.2223-4292.2015.03.01. doi: 10.3978/j.issn.2223-4292.2015.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: Detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998;170:397–402. doi: 10.2214/ajr.170.2.9456953. doi: 10.2214/ajr.170.2.9456953. [DOI] [PubMed] [Google Scholar]
- 22.Shen S, Wang H, Zhang J, Wang F, Liu SR. Diffusion weighted imaging, diffusion tensor imaging, and T2* mapping of lumbar intervertebral disc in young healthy adults. Iran J Radiol. 2016;13:e30069. doi: 10.5812/iranjradiol.30069. doi: 10.5812/iranjradiol.30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Q, Gao X, Brown MD, Temple HT, Gu W. Simulation of water content distributions in degenerated human intervertebral discs. J Orthop Res. 2017;35:147–53. doi: 10.1002/jor.23284. doi: 10.1002/jor.23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kealey SM, Aho T, Delong D, Barboriak DP, Provenzale JM, Eastwood JD, et al. Assessment of apparent diffusion coefficient in normal and degenerated intervertebral lumbar disks: Initial experience. Radiology. 2005;235:569–74. doi: 10.1148/radiol.2352040437. doi: 10.1148/radiol.2352040437. [DOI] [PubMed] [Google Scholar]
- 25.Antoniou J, Demers CN, Beaudoin G, Goswami T, Mwale F, Aebi M, et al. Apparent diffusion coefficient of intervertebral discs related to matrix composition and integrity. Magn Reson Imaging. 2004;22:963–72. doi: 10.1016/j.mri.2004.02.011. doi: 10.1016/j.mri.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Maasumi K, Tehranzadeh J, Muftuler LT, Gardner V, Hasso AN. Assessment of the correlation between apparent diffusion coefficient and intervertebral disk degeneration using 3 tesla MRI. Neuroradiol J. 2011;24:593–602. doi: 10.1177/197140091102400416. doi: 10.1177/197140091102400416. [DOI] [PubMed] [Google Scholar]
- 27.Nazari J, Pope MH, Graveling RA. Feasibility of magnetic resonance imaging (MRI) in obtaining nucleus pulposus (NP) water content with changing postures. Magn Reson Imaging. 2015;33:459–64. doi: 10.1016/j.mri.2015.01.006. doi: 10.1016/j.mri.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Stelzeneder D, Welsch GH, Kovács BK, Goed S, Paternostro-Sluga T, Vlychou M, et al. Quantitative T2 evaluation at 3.0T compared to morphological grading of the lumbar intervertebral disc: A standardized evaluation approach in patients with low back pain. Eur J Radiol. 2012;81:324–30. doi: 10.1016/j.ejrad.2010.12.093. doi: 10.1016/j.ejrad.2010.12.093. [DOI] [PubMed] [Google Scholar]