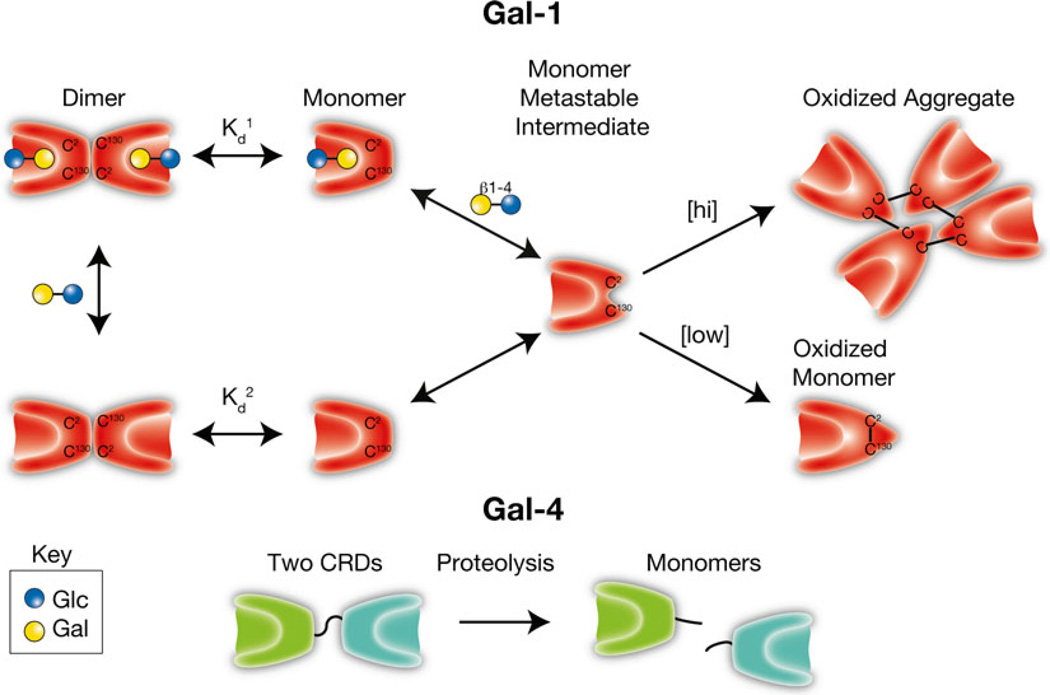
Fig. 7.
Galectin activity is regulated by oxidation and proteolysis. Galectins, first called S-type lectins secondary to the requirement of several galectins for reduced thiols to maintain carbohydrate recognition activity, can form intramolecular and intermolecular disulfide bridges that often result in significant conformational changes that preclude carbohydrate recognition. As monomers appear to be a key intermediate in oxidative inactivation and carbohydrates can drive dimerization, ligand appears to reduce oxidative inactivation by facilitating dimer formation (Kd1 < Kd2). [hi] = higher concentrations of Gal-1. [low] = lower concentrations of Gal-1. In contrast, several galectins, especially tandem repeat and chimeric galectins, rely on linker peptide bound carbohydrate recognition domains or N-terminal collagen-like domains to facilitate dimerization/oligomerization. Cleavage of intervening peptides that connect oligomerization domains to functional carbohydrate recognition domains can render carbohydrate recognition domains monomeric and therefore incapable of generating molecular lattices typically thought to be required for optimal galectin-mediated signaling