Fig. 2. Non-bilayer structures modulate ATP synthase activity in mitochondria.
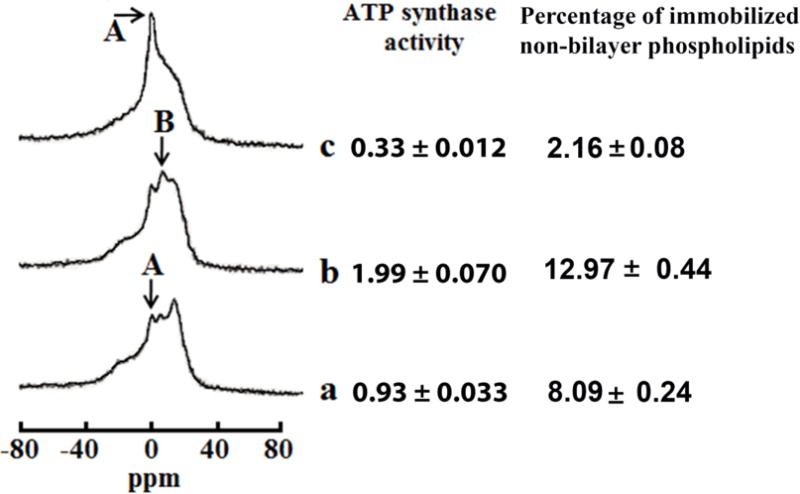
31P-NMR spectra of mitochondria recorded in the presence of 1.5mM succinate at 15 °C in a control sample (a), and in the samples treated with 9 × 10−4 M CTII (b), pre-treated with 9 × 10−4 M CTII and post-treated with 1 × 10−7 M PLA2 (c). The phospholipid concentration in each mitochondrial sample was estimated to be approximately 6.3 × 10−2 M, which was assessed by normalizing the integrated density of the 31P-NMR signals from the mitochondrial samples to the integrated density of the 31P-NMR signals from large multilamellar liposomes. The amount of non-bilayer immobilized phospholipids was estimated by calculating the area under the signal remained after a DANTE train of saturation was applied at the high-field peak of the lamellar spectrum (not shown). The percentage of non-bilayer immobilized phospholipids are shown on the column on the right as means with standard errors (±SEM) compiled from three independent experiments. The ATP levels, expressed as μmol ATP synthesized per mg of mitochondrial proteins, was monitored by taking measurements on aliquots from the 31P-NMR sample tubes. ATP levels are shown on the right column as means with standard errors (±SEM) compiled from three independent experiments. Statistical differences were observed between ATP synthase activity in untreated mitochondria (a) vs. mitochondria treated with CTII (b) (One Way ANOVA, Fisher’s LSD). Each 31P-NMR spectrum shown is representative of two independent experiments that showed similar results. Each sample was measured in triplicate readings.
