Abstract
The technique of sputum induction and processing is a recognized non-invasive method allowing the collection and analysis of cells from the airways, which is interesting in various respiratory diseases like asthma, chronic obstructive pulmonary disease (COPD), chronic cough, or idiopathic pulmonary fibrosis. This technique is well tolerated, safe and non-invasive, but is currently limited to research services and specialized centers in clinical practice because it is technically demanding, time-consuming, and requires trained staff. The success rate of sputum induction and analysis is about 80%.
Here, we describe the induction and laboratory processing of sputum samples. Sputum is induced by inhalation of hypertonic or isotonic saline with salbutamol. For the processing, we use the whole sputum technique. Dithiothreitol (DTT) is used to allow mucolysis of sputum samples. The primary aim of sputum processing is to obtain a differential cell count to study the cell types present in the airway lumen. Additional analyses may also be performed on sputum supernatant and sputum cells, which may allow further investigation into inflammatory processes and immune mechanisms. Examples include studying mediators in sputum supernatant and performing a large spectrum of analysis on sputum cells such as flow cytometry, genomics, or proteomics.
Finally, representative results of sputum analysis in healthy controls, asthmatics, and COPD patients are presented.
Keywords: Medicine, Issue 130, Humans, Sputum, Supernatant, Airway Inflammation, Eosinophils, Neutrophils, Macrophages, Lymphocytes, Epithelial cells, Lung Diseases
Introduction
Several methods are used to investigate airway inflammation: direct measurements (like bronchial biopsies or bronchoalveolar lavages) and indirect methods (like symptom assessment, blood sample analysis and lung function tests)1. The direct techniques have the advantage of reliably assessing the airway inflammation, but they are invasive and not feasible at large scale because of patient discomfort and the risk incurred1. As for the indirect methods, they poorly correlate with the direct assessment of airway inflammation1.
Sputum collection is another way to sample cells from the airways and allows the direct assessment of airway inflammation. Nevertheless, producing sputum spontaneously may lead to samples of poor quality and is not possible for all patients2. This issue has been overcome by using ultrasonically nebulized hypertonic saline to induce the sputum production2. This method was initially used in patients infected with human immunodeficiency virus (HIV) for the diagnosis of Pneumocystis carinii pneumonia3 and was adapted for healthy subjects and asthmatics in 19924. Sputum induction is also feasible in more severe patients by using isotonic saline5. Although generally well-tolerated, the inhalation of saline may cause bronchospasm in patients with hyperresponsive airways5. Therefore, it is recommended to administer a short-acting beta agonist before the procedure1. Moreover, we have previously shown that adding salbutamol to the saline solution in the ultrasonic nebulizer further decreased this risk6. The advantages of sputum induction is that it is non-invasive2 and safe when appropriate precautions are taken5.
For the processing of sputum samples, two methods are currently used in the literature: the whole sputum technique and the plug selection7. In our laboratory, the whole sputum technique is performed. The primary aim of sputum processing is to obtain a differential cell count to study the type of inflammation present in the airway lumen. However, many additional analyses are also possible to further investigate inflammatory processes and immune mechanisms, either by studying mediators in the sputum supernatant8 or by performing detailed investigation on sputum cells (e.g., flow cytometry9, cell cultures10, genomics10, proteomics10, immunocytochemistry7, in situ hybridization7, etc.)
The technique of sputum induction and analysis is currently limited to research services and specialized centers in clinical practice because it is technically demanding, time-consuming, and requires trained staff1. Airway inflammation may be investigated with this method for various respiratory diseases like asthma, chronic obstructive pulmonary disease (COPD), chronic cough, or idiopathic pulmonary fibrosis11.
Protocol
All methods described in this section have been approved by the Ethics Committee of the University Hospital of Liege and all healthy subjects gave written informed consent for participation.
1.Sputum Induction
- Pre- and post-bronchodilator spirometry
- Perform a spirometry (forced expiratory volume in 1 second [FEV1]and forced vital capacity [FVC] maneuver) according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) standard criteria12.
- Administer 400 µg of inhaled salbutamol from a metered dose inhaler (MDI) through a spacer device. NOTE: In adults, adverse events of inhaled salbutamol include tremor and tachycardia13.
- Repeat spirometry (FEV1 and FVC maneuver) 15 min later, according to the ATS/ERS standard criteria12.
- Preparation of the ultrasonic nebulizer NOTE: The nebulizer must be thoroughly cleaned and disinfected before each use, in accordance with the manufacturer's instructions.
- Fill the nebulizer chamber with distilled water up to the recommended level.
- Place a clean cup in the nebulizer chamber.
- Cover the cup with the appropriate lid.
- Fill the cup with either 50 mL of hypertonic saline (5%) for a patient with post-bronchodilator FEV1 >65% predicted or 50 mL of isotonic saline (0.9%) for a patient with post-bronchodilator FEV1≤65% predicted.
- Add 1.75 mL of salbutamol sulfate solution (5 mg/mL) to the cup.
- Connect the tubes and valves in accordance with the manufacturer's instructions.
- Nebulization and sputum collection NOTE: Perform the technique under medical supervision.
- Explain the procedure to the patient.
- Ask the patient to use a nose clip.
- Turn the nebulizer on and select the lowest level of aerosol and fan settings.
- Ask the patient to inhale the aerosol through the mouth piece with tidal breathing for 5 min. NOTE: Aerosol and fan settings may be increased depending on the patient's tolerance.
- In case of unbearable cough or nausea, discontinue the procedure and go to step 1.3.5.
- In the event of chest tightness or respiratory discomfort, go to step 1.3.8.
- Turn the nebulizer off.
- Ask the patient to remove the nose clip, rinse his mouth with water, and gargle twice before discarding it in the sink. NOTE: Saliva cells may contaminate the sputum sample and lead to an unusable sample.
- Ask the patient to cough up sputum into a plastic container.
- Perform a spirometry (forced expiratory maneuver) to measure FEV1.
- Evaluate the FEV1 fall as follows: (FEV1 measured at step 3.8 [mL]) - (post-bronchodilator FEV1 measured at step 1.3 [mL]) / (post-bronchodilator FEV1 measured at step 1.3 [mL])*100.
- If the FEV1 falls by 20% or more from the post-bronchodilator value, discontinue the procedure. Measure FEV1 again 10 min later. If the FEV1 falls 20% or more, ask the physician to administer nebulized ipratropium bromide (0.25 mg/2 mL) and keep the patient under medical observation. Go to step 1.3.10.
- If the FEV1 does not fall by 20% or more from the post-bronchodilator value, repeat steps 3.2 to 3.9 one to three times to reach a total nebulization time of 10 to 20 min. NOTE: The total nebulization time will depend on quality (presence of plugs or viscous parts) and quantity of the sputum sample. If the sample quality and/or quantity is poor after 10 min of nebulization, the procedure should be extended to reach a total nebulization time of 15 to 20 min.
- Keep the sputum sample refrigerated until processing.
2. Sputum Processing
NOTE: Process the sputum within 3 hours of sampling for optimum cell viability.
Caution: Wear a lab coat, protective gloves, and goggles.
- Preliminary Preparations
- Make a 10-fold dilution of the concentrated dithiothreitol solution (mucolytic agent) with sterile distilled water to obtain 10 mL of a 6.5 mM solution, in accordance with the manufacturer's instructions. NOTE: In order to prevent concentrated dithiothreitol solution mixing with ambient air, withdraw the solution from the vial with a sterile syringe and needle. Once opened, the concentrated solution vial must be kept at room temperature and used within 5 days. The diluted solution is prepared daily. CAUTION: Because of eye and skin irritation hazard, wear protective equipment (clothing, gloves, and goggles).
- Make a 5-fold dilution of the trypan blue solution (0.4%) with Dulbecco's Phosphate-Buffered Saline (DPBS) to obtain a 0.08% solution. Keep this diluted solution at room temperature and use within 2 weeks. CAUTION: Because of carcinogenic hazards, wear protective equipment (clothing, gloves and goggles).
- Collection of Sputum Supernatant
- Transfer the whole sputum in a 50 mL conical bottom plastic tube and weigh the sample.
- Add a 3-fold weight of DPBS solution.
- Slowly vortex the sample for 30 s.
- Centrifuge at 800 x g and 4 °C for 10 min.
- Filter the sample through 2 single layers of sterile gauze and collect the supernatant in a 50 mL conical bottom plastic tube.
- Aliquot the supernatant in 2 mL plastic tubes and store them at -80 °C. NOTE: Supernatant samples are useful to assay sputum fluid phase components.
- Sputum Mucolysis
- If necessary, add DPBS to the cell pellet to reach a total volume of cell suspension of 5 mL.
- Dilute the cell suspension with 1 volume of 6.5 mM dithiothreitol. Use a bench rocker to rock the cell suspension for 20 min at room temperature.
- Repeat at least 3 times with DPBS.
- Centrifuge the diluted cell suspension at 550 x g and 4 °C for 10 min. Discard the supernatant.
- Resuspend the cell pellet in around 1 mL of DPBS.
- Assessment of qualitative properties of the sputum sample (cell concentration, squamous cell contamination, and viability by Trypan Blue exclusion method) by hemocytometer count
- Assess and record the exact volume of the cell suspension.
- Add 50 µL of cell suspension to 50 µL of the 0.08% trypan blue solution and homogenize.
- Put the sample under the coverslip of a hemocytometer (Thoma chamber) and place it under the optical microscope (400X).
- Count the squamous cells, the living non-squamous cells (uncolored cells), and the dead non-squamous cells (blue colored cells). NOTE: In case of too low or too high cell density, concentrate or dilute the cell suspension and repeat steps 2.4.1-2.4.3.
- Assess the cell concentration of the suspension, the percentage of squamous cells, and the percentage of viability of non-squamous cells. NOTE: Calculate the total non-squamous cell count/g of sputum: cell concentration of the suspension (step 5.4) * volume of the cell suspension (step 4.8) *% of non-squamous cells / weight of the sputum sample (step 2.2.1).
- Preparation of a stained cytospin slide with the cell suspension and storage of residual cells, if needed NOTE: If the cell suspension contains more than 80% squamous cells, the sample is considered poor quality and unsuitable for a cytospin slide14. In that case, discontinue the processing and consider this sample unsuccessful.
- Dilute an aliquot of the cell suspension with DPBS to obtain at least 350 µL of a cell suspension at 500,000 cells/mL.
- Assemble the cell concentrator in accordance with the manufacturer's instructions.
- Fill each of the 3 compartments of the cell concentrator with 100 µL of this cell suspension.
- In a cytocentrifuge, spin for 2 min at 150 x g and room temperature. Discard the supernatants.
- Disassemble the cell concentrator in accordance with the manufacturer's instructions.
- Allow the slide to air dry. NOTE: At this stage, residual cells can be stored in an adequate buffer if needed for further experiments.
- Stain the slide with a commercial staining kit (see the Table of Materials), in accordance with the manufacturer's instructions.
- Mount the slide with a suitable mounting medium and a cover slip.
- Place the slide under the optical microscope (600X) with oil immersion.
- Count at least 500 non-squamous cells: neutrophils, eosinophils, macrophages, lymphocytes, and epithelial cells. NOTE: Differential cell count is usually performed by only one or two dedicated and trained observers to limit interobserver discrepancies.
- Record absolute values and percentages of each cell type.
Representative Results
Hemocytometer A typical image is shown in Figure 1 that is visualized with the microscope using the hemocytometer. Squamous cells are easily identified, as they are much larger than the non-squamous cells. These squamous cells are epithelial cells coming from the mouth. Both types of cells are stained by Trypan Blue when dead. Caution must be taken to avoid counting yeast, bacteria, and scrap.
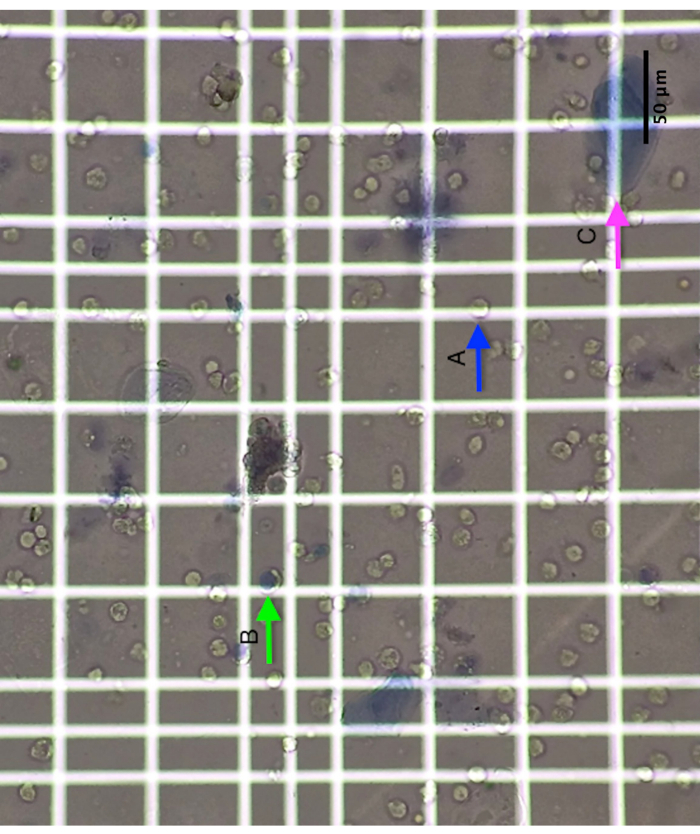
Figure 1: Hemocytometer picture showing (A) a living non-squamous cell, (B) a dead non-squamous cell, and (C) a squamous cell. Scale bar = 50 µm. Please click here to view a larger version of this figure.
Cytospin slide: Figure 2 shows a representative image of a cytospin slide obtained after sputum processing. The different cell types (neutrophils, eosinophils, macrophages, lymphocytes, and epithelial cells) can be differentiated by means of their morphology and coloration. In some cases, contamination by squamous cells may be important and, if the percentage of squamous cells is greater than 80%, the sample is considered unsuccessful (Figure 3).
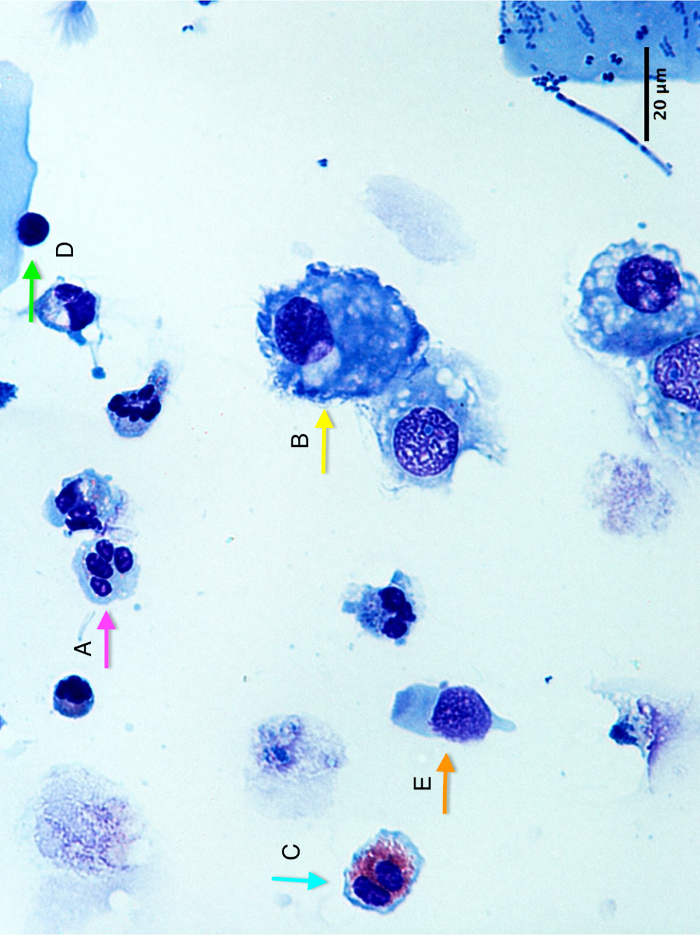
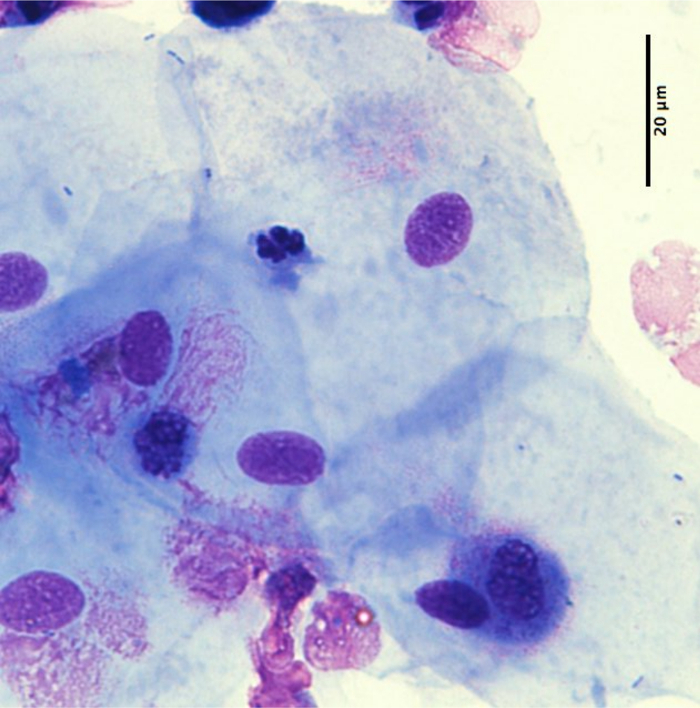
Figure 2: Cytospin slide picture showing (A) a neutrophil, (B) a macrophage, (C) an eosinophil, (D) a lymphocyte, and (E) an epithelial cell. Scale bar = 20 µm. Please click here to view a larger version of this figure.
Figure 3: Example of a poor quality cytospin slide with >80% of squamous cells. Scale bar = 20 µm. Please click here to view a larger version of this figure.
Success rate In our department, the success rate of the procedure (combining a successful induction and a readable cytospin), based on a sample of 1,129 patients (healthy subjects, asthmatics, or COPD patients), is 82% (924/1,129). In a sub-analysis according to the type of patients, the success rate is 75% (57/76) in healthy subjects, 82% (827/1,004) in asthmatics, and 82% (40/49) in COPD patients.
Results in healthy subjects In a retrospective analysis of a series of 289 healthy subjects from our department, the median (interquartile range) sputum weight was 3.72 g (interquartile range of 2.46 g - 5.54 g) and the median total non-squamous cell count/g of sputum was 0.59 x 106 (interquartile range of 0.37 x 106 - 1.29 x 106).
In those healthy subjects, the proportion of squamous cells is low at 19% (10%-34%) and the viability is high at 66% (54-78%). Regarding the percentage of the different cell types, results are summarized in Figure 4A. We can observe that the percentage of macrophages (49% [31-68%]) is higher than the percentage of neutrophils (34% [14%-60%]), while the percentages of lymphocytes (2% [1-3%]), eosinophils (0% [0%-0%]) and epithelial cells (4% [2-11%]) are low. These results are similar when data are expressed in absolute values (Figure 4B).
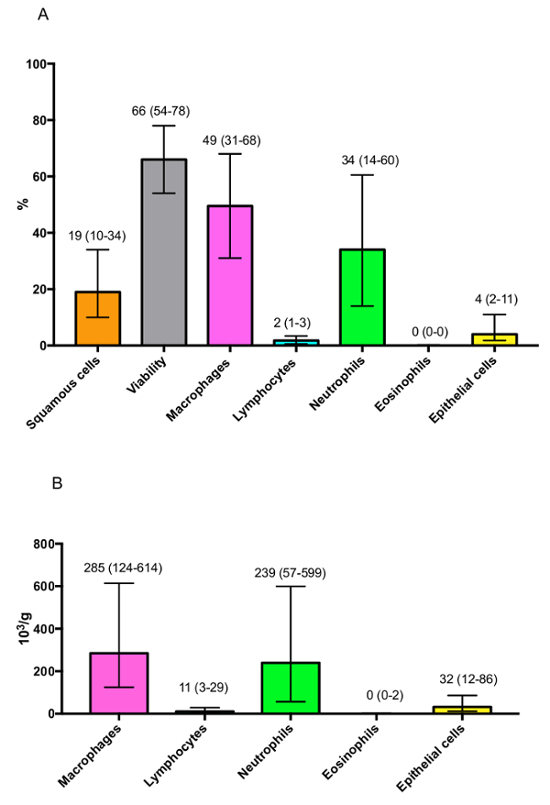
Figure 4: Representative results of the differential cell count observed in healthy subjects expressed as (A) percentages or (B) absolute values. Results are shown as median (interquartile range). Please click here to view a larger version of this figure.
It is also important to take the age of patients into consideration. Indeed, a strong correlation is present between a patient's age and the proportion of neutrophils in sputum samples (Figure 5). Likewise, when classifying patients according to 10 year age groups (Figure 6), we observed a significant increase in the neutrophil percentage with increasing age group. Therefore, this variable must be considered when comparing results from different cohorts, and caution must be taken in the matching of subjects.
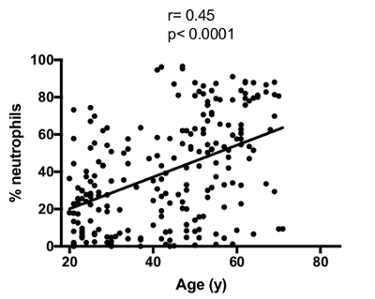
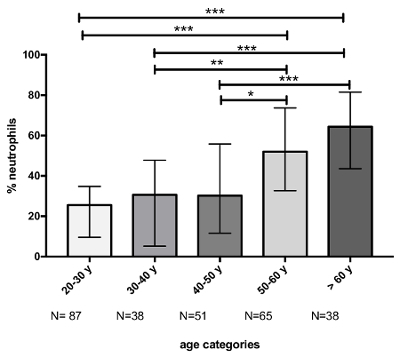
Figure 5: Correlation between age and sputum neutrophil percentage. The correlation was calculated with the Spearman test. Please click here to view a larger version of this figure.
Figure 6:Evolution of the neutrophil percentage according to the age category. The p-value of ANOVA was <0.0001 for the comparison of neutrophil percentage between age classes. Multiple comparisons were made with Dunn's multiple comparisons test. The p-values are represented as follows: * p <0.05, ** p <0.01, and *** p <0.001. Results are shown as median (interquartile range). Please click here to view a larger version of this figure.
Results in patients suffering from respiratory diseases The technique of induced sputum is commonly used to assess the inflammatory cell profile in asthmatic patients. This technique may also be applied to patients suffering from COPD, another inflammatory respiratory disease. When comparing healthy subjects, asthmatics, and COPD patients (the 3 groups being matched for age, gender, and tobacco habits), we observed that the inflammatory cell profile is quite different between these cohorts (Figure 7). Indeed, asthmatic patients are usually characterized by raised sputum eosinophils, while the proportion of sputum neutrophils is usually higher in COPD patients compared to healthy controls, which is linked to the disease severity.
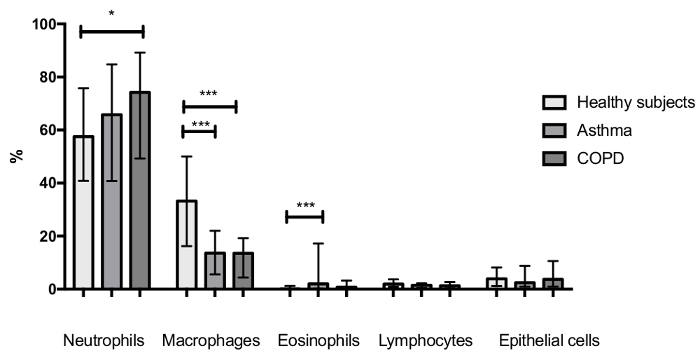
Figure 7:Sputum inflammatory cell profile of healthy subjects (n = 45), asthmatic patients (n = 108), and COPD patients (n = 54). The three groups were matched for gender, age, and tobacco habits. The p-values of ANOVA were <0.05, <0.0001, and <0.0001 for the comparison of neutrophils, eosinophils, and macrophages between groups, respectively. Multiple comparisons were made with Dunn's multiple comparisons test. The p-values are represented as follows: * p <0.05 and *** p <0.001. Results are shown as median (interquartile range). Please click here to view a larger version of this figure.
Discussion
The induced sputum method is a useful tool to study the airway compartment. There are several possible applications of this technique. First, it may improve knowledge about immune cells and mechanisms involved in various respiratory diseases. For example, this technique has allowed the investigation of airway inflammation in large cohorts of patients, and it has been shown that about half of asthmatic patients are characterized by an abnormal airway eosinophilic inflammation15,16,17, while COPD patients usually exhibit a raised sputum neutrophil count18. This technique has also contributed to better characterization of airway inflammation in patients with idiopathic pulmonary fibrosis and to evidence mediators that may contribute to this disease19. Second, the technique of induced sputum may be useful to predict response to treatment. For example, the presence of an abnormal percentage of sputum eosinophils has been shown to be a predictive marker of corticosteroid responsiveness11,18. In asthma and COPD, tailoring the dose of corticosteroids to normalize the percentage of sputum eosinophils was shown to be more effective in decreasing the number of exacerbations than adjusting treatment according to the current clinical guidelines11,20,21. Third, sputum analysis may help to develop targeted therapies. For example, the presence of an abnormal number of sputum neutrophils in COPD and some asthmatic patients has led to the development of antineutrophilic treatments11. Fourth, the technique may play a role in diagnosis. For example, the presence of sputum eosinophilia is necessary to make a diagnosis of non-asthmatic eosinophilic bronchitis11.
Besides obtaining a differential cell count, the technique of inducing sputum also allows the performance of many additional analyses, by studying either sputum supernatant or sputum cells. Examples with sputum supernatant include the analysis of mediators8,19,22 and the assessment of the chemotactic activity of the sample for eosinophils22. From sputum cells, RNA can be extracted and used for microRNA or gene expression analyses19,23 . The sputum cells can also be analyzed by flow cytometry9,23 which allows, among others, immunophenotyping and sorting cells. Furthermore, sputum cells can be cultured10, and their mediator production can be measured in vitro24. It is worth noting that in this case, the mediator content is different from what can be found in sputum supernatant. Indeed, mediators in sputum supernatant may be influenced by secretions from airway resident cells and by plasma exudation, contrary to the sputum cell culture model24. Finally, immunocytochemistry and in situ hybridization can also be performed using sputum cells7.
The induced sputum technique has some limitations. The induction must be performed under medical supervision. It is also essential for the operator to give thorough instructions to patients. Other limitations include the cooperation and medical condition of patients, as respiratory efforts are needed. Regarding the feasibility of this technique in children, several studies have reported that it was successful and safe in children older than 6 years old25. Data on children of <6 years of age are lacking25, but it is likely that sputum induction is difficult to perform in those children14. The technique is currently restricted to research services and specialized centers because it is technically demanding, time-consuming, and it requires trained staff1. Another limitation is that the technique of sputum induction and analysis is not always successful, meaning that a readable cytospin is not always obtained26. However, the success rate is usually around 80% in different cohorts of patients4,26,27,28,29. Finally, caution must be taken when comparing different cohorts of patients regarding the matching of age as it was shown to impact the sputum cell count30,31. Likewise, other parameters such as gender and tobacco habits have to be matched as they may also interfere with the sputum cell count29,32. When patient matching is not possible, another way to take age, gender or smoking status into account is to adjust the statistical analysis for these characteristics.
Compared to other techniques that allow collecting cells from airways, like biopsies and bronchoalveolar lavages, the induced sputum method has the advantages of being simple, well-tolerated, safe, reproducible, cost-effective, and non-invasive. These advantages allow the induced sputum technique to be performed at a large scale and repeatedly over time, and make it an alternative of choice for airway sampling. Bronchosorption is another technique allowing the collection of the mucosal lining fluid in order to assess airway mediators33. While this technique (requiring bronchoscopy) is more invasive than induced sputum, it has the advantages of avoiding any contamination with saliva and obtaining higher concentrations of mediators than in bronchoalveolar lavage33.
Critical steps need attention in the protocol. First, caution must be taken concerning the saline concentration and the time of induction, because these two parameters can influence the results and therefore have to be standardized34,35. Second, the mucolysis with DTT is an important step of the processing. As compared with PBS, DTT was shown to better disperse sputum cells7, which improves the cytopsin slide quality and is essential to have a reproducible cell count2. However, the mucolytic agent may interfere with the measurement of biochemical components. Spiking experiments should then be performed when measuring a new biochemical compound36. Finally, another critical step of the procedure is the cell counting step, which must be performed by a trained technician to ensure reliable and interpretable results.
An alternative method using sputum plugs (viscid or denser parts), instead of the whole sputum, is also described in the literature7. Both techniques have advantages and disadvantages. The whole sputum technique allows more rapid processing7, but is frequently contaminated with saliva, which dilutes the sample and can reduce the quality of cytospins2,7. With the plug selection technique, the saliva contamination is reduced, which means that samples are often of better quality for the dosage of fluid phase mediators and for cytospin slides7. The processing is however longer7 and selected plugs may not be representative of the whole sample37. It is also worth noting that not all samples contain plugs, which can be limiting. Both methods are validated and reproducible, and there is currently no evidence that the differential cell counts obtained from both these methods are different14,38.
In the future, induced sputum could be used as a clinical tool to provide biomarkers for the investigation, diagnosis, and management of all kinds of inflammatory respiratory diseases.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the University Hospital of Liege (CHU Liege). We acknowledge "Instants productions" for the video production. We also acknowledge Cedric François, the patient who appeared in the film.
References
- Jayaram L, Parameswaran K, Sears MR, Hargreave FE. Induced sputum cell counts: their usefulness in clinical practice. European Respiratory Journal. 2000;16(1):150–158. doi: 10.1034/j.1399-3003.2000.16a27.x. [DOI] [PubMed] [Google Scholar]
- Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52(6):498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh TR, et al. Sputum induction for diagnosis of Pneumocystis carinii pneumonia. The Lancet. 1989;334(8656):205–206. doi: 10.1016/s0140-6736(89)90382-6. [DOI] [PubMed] [Google Scholar]
- Pin I, et al. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax. 1992;47(1):25–29. doi: 10.1136/thx.47.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzichini MMM, Leigh R, Djukanović R, Sterk PJ. Safety of sputum induction. European Respiratory Journal. 2002;20(37 suppl):9s–18s. doi: 10.1183/09031936.02.00000902. [DOI] [PubMed] [Google Scholar]
- Delvaux M, et al. Nebulised salbutamol administered during sputum induction improves bronchoprotection in patients with asthma. Thorax. 2004;59(2):111–115. doi: 10.1136/thorax.2003.011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efthimiadis A, et al. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. European Respiratory Journal. 2002;20(37 suppl):19s–23s. doi: 10.1183/09031936.02.00001902. [DOI] [PubMed] [Google Scholar]
- Kelly M, et al. Analysis of fluid-phase mediators. European Respiratory Journal. 2002;20(37 suppl):24s–39s. [PubMed] [Google Scholar]
- Lay JC, Peden DB, Alexis NE. Flow cytometry of sputum: assessing inflammation and immune response elements in the bronchial airways. Inhalation Toxicology. 2011;23(7):392–406. doi: 10.3109/08958378.2011.575568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignola AM, et al. Future directions. European Respiratory Journal. 2002;20(37 suppl):51s–55s. [PubMed] [Google Scholar]
- Brightling CE. Clinical applications of induced sputum. Chest. 2006;129(5):1344–1348. doi: 10.1378/chest.129.5.1344. [DOI] [PubMed] [Google Scholar]
- Miller MR. Standardisation of spirometry. European Respiratory Journal. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. 2017. http://www.ginasthma.org/
- Szefler SJ, et al. Asthma outcomes: biomarkers. The Journal of Allergy and Clinical Immunology. 2012;129(3 Suppl):S9–S23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57(7):643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleich FN, et al. Importance of concomitant local and systemic eosinophilia in uncontrolled asthma. The European Respiratory Journal. 2014;44(1):97–108. doi: 10.1183/09031936.00201813. [DOI] [PubMed] [Google Scholar]
- Demarche S, et al. Detailed analysis of sputum and systemic inflammation in asthma phenotypes: are paucigranulocytic asthmatics really non-inflammatory? BMC pulmonary medicine. 2016;16:46. doi: 10.1186/s12890-016-0208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavord ID, et al. Clinical applications of assessment of airway inflammation using induced sputum. The European Respiratory Journal. Supplement. 2002;37:40s, 43s. doi: 10.1183/09031936.02.00004002. [DOI] [PubMed] [Google Scholar]
- Guiot J, Henket M, Corhay JL, Moermans C, Louis R. Sputum biomarkers in IPF: Evidence for raised gene expression and protein level of IGFBP-2, IL-8 and MMP-7. PLOS ONE. 2017;12(2):e0171344. doi: 10.1371/journal.pone.0171344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RH, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- Siva R, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. European Respiratory Journal. 2007;29(5):906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- Louis R, et al. Cell infiltration, ICAM-1 expression, and eosinophil chemotactic activity in asthmatic sputum. American Journal of Respiratory and Critical Care Medicine. 1997;155(2):466–472. doi: 10.1164/ajrccm.155.2.9032180. [DOI] [PubMed] [Google Scholar]
- Maes T, et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum. The Journal of Allergy and Clinical Immunology. 2016;137(5):1433–1446. doi: 10.1016/j.jaci.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Moermans C, et al. Local and systemic cellular inflammation and cytokine release in chronic obstructive pulmonary disease. Cytokine. 2011;56(2):298–304. doi: 10.1016/j.cyto.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Gibson PG, et al. Sputum induction in children. European Respiratory Journal. 2002;20(37 suppl):44s–46s. doi: 10.1183/09031936.02.00004402. [DOI] [PubMed] [Google Scholar]
- Reddel HK, et al. An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and Exacerbations: Standardizing Endpoints for Clinical Asthma Trials and Clinical Practice. American Journal of Respiratory and Critical Care Medicine. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- Duncan CJA, Lawrie A, Blaylock MG, Douglas JG, Walsh GM. Reduced eosinophil apoptosis in induced sputum correlates with asthma severity. The European Respiratory Journal. 2003;22(3):484–490. doi: 10.1183/09031936.03.00109803a. [DOI] [PubMed] [Google Scholar]
- Demarche SF, et al. Asthma Control and Sputum Eosinophils: A Longitudinal Study in Daily Practice. The Journal of Allergy and Clinical Immunology: In Practice. 2017;5(5):1335–1343. doi: 10.1016/j.jaip.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Belda J, et al. Induced sputum cell counts in healthy adults. American Journal of Respiratory and Critical Care Medicine. 2000;161(2 Pt 1):475–478. doi: 10.1164/ajrccm.161.2.9903097. [DOI] [PubMed] [Google Scholar]
- Brooks CR, Gibson PG, Douwes J, Dalen CJV, Simpson JL. Relationship between airway neutrophilia and ageing in asthmatics and non-asthmatics: Airway neutrophilia and ageing. Respirology. 2013;18(5):857–865. doi: 10.1111/resp.12079. [DOI] [PubMed] [Google Scholar]
- Thomas RA, et al. The influence of age on induced sputum differential cell counts in normal subjects. Chest. 2004;126(6):1811–1814. doi: 10.1378/chest.126.6.1811. [DOI] [PubMed] [Google Scholar]
- Chalmers GW, et al. Smoking and airway inflammation in patients with mild asthma. Chest. 2001;120(6):1917–1922. doi: 10.1378/chest.120.6.1917. [DOI] [PubMed] [Google Scholar]
- Hansel TT, et al. A Comprehensive Evaluation of Nasal and Bronchial Cytokines and Chemokines Following Experimental Rhinovirus Infection in Allergic Asthma: Increased Interferons (IFN-γ and IFN-λ) and Type 2 Inflammation (IL-5 and IL-13) EBioMedicine. 2017;19:128–138. doi: 10.1016/j.ebiom.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov TA, et al. Some technical factors influencing the induction of sputum for cell analysis) European Respiratory Journal. 1995;8(4):559–565. [PubMed] [Google Scholar]
- Holz O, et al. Changes in sputum composition between two inductions performed on consecutive days. Thorax. 1998;53(2):83–86. doi: 10.1136/thx.53.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis R, et al. The effect of processing on inflammatory markers in induced sputum. European Respiratory Journal. 1999;13(3):660–667. doi: 10.1183/09031936.99.13366099. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Liu J, Wong H, Boushey HA. Cellular and Biochemical Analysis of Induced Sputum from Asthmatic and from Healthy Subjects. American Review of Respiratory Disease. 1993;147(5):1126–1131. doi: 10.1164/ajrccm/147.5.1126. [DOI] [PubMed] [Google Scholar]
- Pizzichini E, Pizzichini MM, Efthimiadis A, Hargreave FE, Dolovich J. Measurement of inflammatory indices in induced sputum: effects of selection of sputum to minimize salivary contamination. The European Respiratory Journal. 1996;9(6):1174–1180. doi: 10.1183/09031936.96.09061174. [DOI] [PubMed] [Google Scholar]


