Table 2.
Substrate scope with new sulfenylating agents.
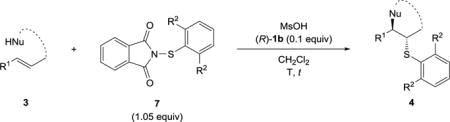
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Alkene 3 | Sulfenylating agent 7 | Product 4 | Temp [°C] | Time [h] | Yield [%]e | e.r.i | |||
| 1 |

|
3a |

|
7a |

|
4aab | −20 | 36 | 78 | 95.6:4.4k |
| 2 |

|
3a |
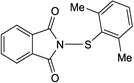
|
7f |

|
4afa | −20 | 36 | 84 | 97.6:2.4k |
| 3 |

|
3a |

|
7h |

|
4aha | −20 | 36 | 87f | 98.4:1.6j,k |
| 4 |

|
3a |

|
7b |

|
4aba | 0 | 48 | 87 g | 99.3:0.7j,k |
| 5 |
|
3g |

|
7a |

|
4gab | 0 | 36 | 83 | 88.9:11.1k |
| 6 |
|
3g |

|
7b |

|
4gba | 0 | 36 | 94 | 98.6:1.4l |
| 7 |
|
3b |
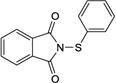
|
7a |

|
4bab, c | −20 | 48 | 77 | 97.4:2.6l |
| 8 |
|
3b |

|
7b |

|
4bba, c | 0 | 36 | 85 | 99.2:0.8l |
| 9 |
|
3e |

|
7a |

|
4eab, c | 23 | 24 | 75 h | 87.8:12.2l |
| 10 |
|
3e |

|
7b |

|
4eba, c | 23 | 24 | 84h | 98.6:1.4l |
| 11 |
|
3h |

|
7a |

|
3haa, d | 0 | 48 | 85 | 83.7:16.3k |
| 12 |
|
3e |

|
7b |

|
3hba, d | 23 | 24 | 72 | 94.3:5.7k |
General reaction conditions: 3 (1.0 mmol), 7 (1.0 mmol), MsOH (1.0 mmol), (R)-1b (10 mol%), CH2Cl2 (0.2 M).
Reaction of literature described compounds21 conducted on 0.2 mmol scale.
MeOH used as external nucleophile.
0.5 equiv MsOH used.
Yield of isolated products.
Combined yield of a 97:3 mixture of tetrahydropyran/-furan.
Combined yield of a 93:7 mixture of tetrahydropyran/-furan.
Combined yield of a 4:1 mixture of 4e and its constitutional isomer (Supporting Information).
Absolute configuration assigned by comparison and in analogy to literature described compounds.
e.r. of the major isomer.
Determined by CSP-SFC analysis.
Determined by HPLC analysis.
