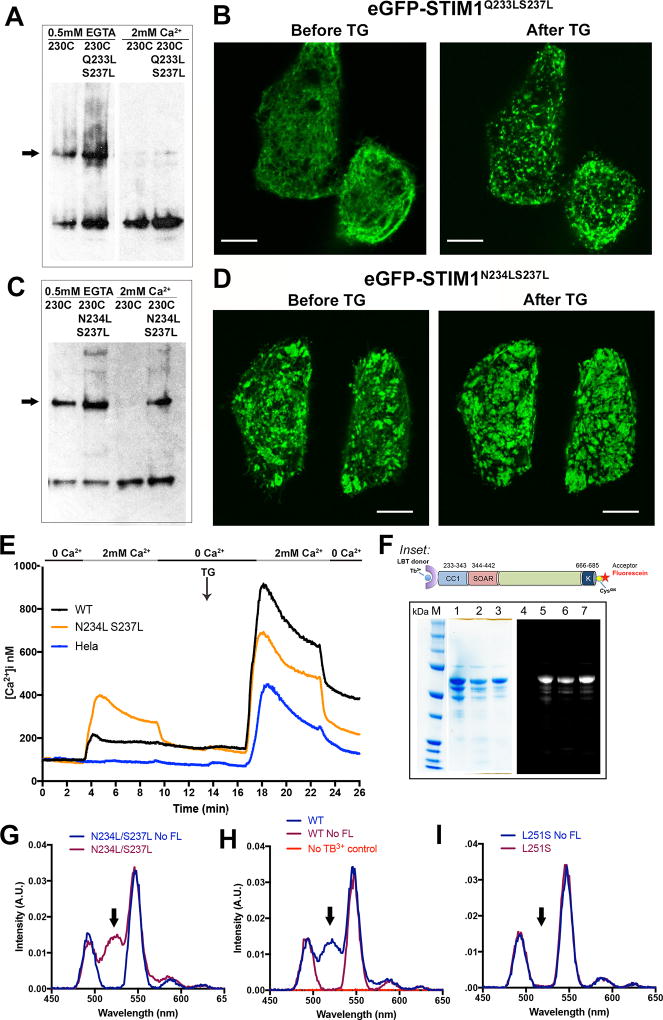
Figure 6. Analysis of STIM1 residues Q233, N234, and S237.
A, The effect of additional Q233L/S237L mutations on crosslinking of STIM1(A230C), assessed as in Figure 2. The STIM dimer band is marked with an arrow. Data representative of three biological replicates. B, Confocal micrographs of HeLa cells expressing GFP-STIM1(Q233L/S237L) under the conditions indicated. Scale bars, 10 µm. C, The effect of additional N234L/S237L mutations on crosslinking of STIM1(A230C), assessed as in Figure 2. The STIM dimer band is marked with an arrow. D, Confocal micrographs of HeLa cells expressing GFP-STIM1(N234L/S237L) under the conditions indicated. Scale bar, 10 µm. E, Single-cell [Ca2+]i measurements in HeLa cells expressing wildtype GFP-STIM1 (WT; n = 55), GFP-STIM1(N234L/S237L) (N234L S237L; n = 73), and non-transfected HeLa cells (HeLa; n = 65). Cells were exposed to solutions containing varied concentrations of CaCl2 or 1 µM thapsigargin (TG) as indicated. These experiments were performed together with those of Figure 4F, and the data plotted in Figure 4F for wildtype GFP-STIM1 and non-transfected HeLa cells are repeated here for reference. F, SDS-polyacrylamide gel analysis of the proteins used for energy transfer experiments. In the gel on the left, the samples in lanes 1–3 are the wildtype, L251S, and N234L/S237L proteins, respectively, stained with Coomassie Brilliant Blue. In the separate gel on the right, the sample in lane 4 is unlabeled wildtype protein as a negative control for fluorescence, and the samples in lanes 5–7 are the same fluorescein-labeled proteins as in lanes 1–3 of the Coomassie Brilliant Blue-stained gel. The gel was illuminated with UV light and the resulting fluorescence imaged to detect dye-labeled proteins. Inset: STIM1 proteins designed for Tb3+-acceptor energy transfer measurements comprise the STIM1 cytoplasmic domain, STIM1(233–685), with an engineered lanthanide-binding tag (LBT) at the N terminus to bind Tb3+ and a single engineered cysteine at the C terminus for covalent labelling with an acceptor dye. Fluorescein-5-maleimide was the labelling reagent in these experiments. G–I, Gated luminescence emission spectra of N234L/S237L (G), wildtype (H), and L251S (I) proteins labeled with donor Tb3+ and acceptor fluorescein. The spectrum of each protein labeled with donor Tb3+, but without acceptor, is shown for comparison (No FL). In (H), the specificity control with acceptor-labeled protein, but omitting Tb3+, is also shown. The arrows in G–I mark the expected position of the fluorescein emission peak. Data are representative of two biological replicates. See also Figure S6.