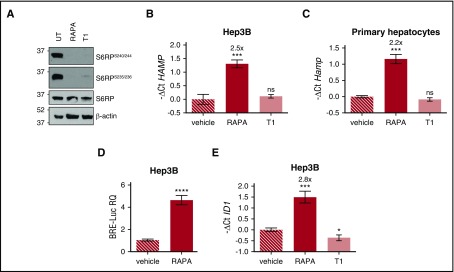
Figure 1.
Rapamycin upregulates hepcidin through activation of the BMP-SMAD pathway. (A) Hep3B cells, treated with rapamycin (RAPA, 100 nM) or Torin1 (T1, 100 nM) for 15 hours, were lysed and whole-cell extract loaded onto a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for western blot analysis. mTOR activation was detected by following Ser240/244 and Ser235/236 phosphorylation of the mTOR target protein S6RP. Total S6RP and actin were analyzed for normalization of gel loading. Molecular weight markers are indicated on the left. A representative western blot, made in triplicate, is shown. (B,E) Hep3B cells were treated with RAPA or T1 as described in panel A. Total RNA was isolated and analyzed by qRT-PCR for hepcidin (HAMP) (B) and ID1 (E) expression. GAPDH was used as a housekeeping gene. A representative experiment, made in triplicate, is shown. (C) Primary murine hepatocytes were treated with RAPA (100 nM) or T1 (100 nM) for 5 hours. RNA was isolated and qRT-PCR was performed to analyze hepcidin (Hamp) expression. Hprt1 was used as housekeeping gene. A representative experiment, made in triplicate, is shown. Mean ΔCt values in each group were subjected to a change of origin by subtracting the mean ΔCt of vehicle-treated cells (B: 7.1; C: −0.83; E: 0.34). (D) Hep3B cells were transfected with the BRE-Luc reporter vector and treated with RAPA (100 nM) as described in panel A. Cells were lysed and analyzed for the luciferase activity that was normalized to an untreated mean value of 1. A representative experiment, made in triplicate, is shown. Error bars indicate SD. *P < .05; **P < .01; ***P < .001; ****P < .0001. Estimates of the fold changes in gene expression (2−ΔΔCt) are shown in the graphs. RQ, relative quantification; ns, not significant.