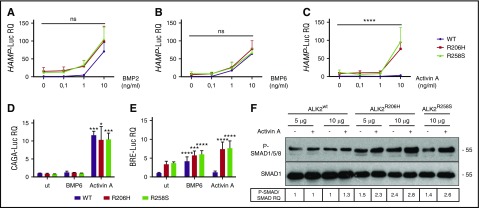
Figure 6.
ALK2-FKBP12–resistant mutants activate hepcidin through Activin A. Hep3B cells were transfected with the hepcidin promoter luciferase reporter vector (HAMP-Luc) and ALK2wt-MYC (purple line), ALK2R206H-MYC (red line), or ALK2R258S-MYC (green line) and treated for 15 hours with increasing concentrations of BMP2 (A), BMP6 (B), and Activin A (C). Cells were lysed and analyzed for the luciferase activity that was normalized to an untreated ALK2wt-MYC mean value of 1. Hep3B cells were transfected with the SMAD2/3 reporter vector (CAGA-Luc) (D) or the SMAD1/5/8 reporter vector (BRE-Luc) (E) in the presence of ALK2wt-MYC, ALK2R206H-MYC, or ALK2R258S-MYC. When indicated, cells were incubated for 15 hours with BMP6 (1 ng/mL) or Activin A (10 ng/mL). Luciferase activity was normalized to an untreated-ALK2wt-MYC mean value of 1. (F) SMAD1/5/8 phosphorylation was analyzed in HuH7 transfected with the Smad1FLAG-expressing vector and 5 or 10 μg of ALK2wt-MYC, ALK2R206H-MYC, or ALK2R258-MYC. When indicated, cells were treated for 15 hours with 10 ng/mL Activin A. Whole cell extract, loaded onto a 10% SDS-PAGE, was analyzed by western blot. SMAD1 and phospho-SMAD1/5/8 were detected using anti-SMAD1 and anti-phosphoSMAD1/5/8 antibodies. A representative western blot, made in triplicate, is shown. Molecular weight markers are indicated on the right. Error bars indicate SD. Two-way ANOVA was used in panels A-C (ALK2 wt vs ALK2 mutants). *P < .05; ***P < .001; ****P < .0001.