Abstract
With the increase in numbers of joint replacements, spinal surgeries, and dental implantations, there is an urgent need to combat implant-associated infection. In addition to stringent sterile techniques, an efficacious way to prevent this destructive complication is to create new implants with antimicrobial properties. Specifically, these implants must be active in the dental implant environment where the implant is bathed in the glycoprotein-rich salivary fluids that enhance bacterial adhesion, and propagation, and biofilm formation. However, in designing an antimicrobial surface, a balance must be struck between antimicrobial activity and the need for the implant to interact with the bone environment. Three types of surfaces have been designed to combat biofilm formation, while attempting to maintain osseous interactions: 1) structured surfaces where topography, usually at the nanoscale, decreases bacterial adhesion sufficiently to retard establishment of infection; 2) surfaces that actively elute antimicrobials to avert bacterial adhesion and promote killing; and 3) surfaces containing permanently bonded agents that generate antimicrobial surfaces that prevent long-term bacterial adhesion. Both topographical and elution surfaces exhibit varying, albeit limited, antimicrobial activity in vitro. With respect to covalent coupling, we present studies on the ability of the permanent antimicrobial surfaces to kill organisms while fostering osseointegration. All approaches have significant drawbacks with respect to stability and efficacy, but the permanent surfaces may have an edge in creating a long-term antibacterial environment.
Keywords: implant-associated infection, antibiotics, antibacterial surfaces, dental implants, biofilm, osseointegration
Implants and Infections
Perhaps the earliest known example of a successful implantation with osseointegration is from the first or second century AD, when an iron tooth was found ankylosed to the jaw of a skeleton (Crubzy et al. 1998). Not surprisingly, the popularity of this procedure has dramatically increased in the past 20 y due to availability of highly engineered titanium, development of aseptic surgical techniques, pain management, and pathogen control via antibiotic treatments. Implants in medical use now range from pacemakers and drug infusion pumps to catheters and dental and orthopaedic devices. Implants with primarily mechanical function, exemplified by those used in dentistry and orthopaedics, have enjoyed considerable success and allow retention of function in an aging population.
For both dental and orthopaedic implants, aseptic loosening and infection are major reasons for failure (Parvizi et al. 2008). As of 2015, about 7 million individuals in the United States have received joint replacements (Maradit Kremers et al. 2015). Within this group, infections occur in approximately 1.5% of total knee replacements and 1.6% of total hip replacements (Kurtz et al. 2010). Treatments involve extended antibiotic therapy, replacement of the infected implant, and at least 1 additional surgery. Reinfection rates can be considerably higher, with estimates in the literature ranging between 26% and 49% (Parvizi et al. 2008).
In dentistry, ~3 million people are estimated to have dental implants, with that number increasing by 500,000/y (American Academy of Implant Dentistry 2017). In terms of individual implant sites, the incidence of peri-mucositis has been reported to be >50% and of peri-implantitis, >12% (Lindhe et al. 2008); the rate of confirmed implant infection has been reported to be ≤2.1% (Powell et al. 2005). As long as bone loss is not severe, appropriate antimicrobial treatment is undertaken, and there is attention to oral hygiene, the dental implant can remain functional (Blus et al. 2015). Although the rate of infection is very low, because of the large number of patients/procedures performed each year, new antimicrobial implant materials/therapies are urgently required.
Some intractable problems are common to both the infected orthopaedic and dental implant. The first is due to the fact that, in both cases, the implant surface has been engineered to integrate with bone. Integration is facilitated by adhesion of proteins to the implant surface. However, this protein deposition generates an ideal surface for bacterial colonization and biofilm formation. In addition, a fibrous layer is formed around parts of the implant, providing a protected environment for bacterial colonization. Finally, the immune response is often attenuated in the presence of this large foreign body (Hickok and Shapiro 2012). In light of these, it is surprising how few implants actually develop a biofilm.
In addition to interfacing with bone in the mandible or maxilla, the dental implant passes through the soft tissue and is exposed to the oral cavity. Within the periodontium, the interface with the percutaneous implant occurs through a fibrous matrix (Singh 2011). Unlike the essentially sterile, orthopaedic implant environment, the intraoral portion of the dental implant is coated by gingival crevicular fluid, food debris, saliva, sugars, and bacterial metabolites. Bacterial colonization is rapid and reflective of the native microbiota. Dental implant infections are usually associated with the Gram-negative periodontal pathogens (Porphyromonas gingivalis, Prevotella intermedial/Prevotella nigrescens, and Actinobacillus actinomycetemcomitans) (Leonhardt et al. 1999), as well as Bacteroides forsythus, Fusobacterium nucleatum, Campylobacter, Peptostreptococcus micros, and Prevotella intermedia. Similar to orthopaedic implants, Staphylococcus spp. (the most common cause of periprosthetic joint infections (Aggarwal et al. 2014), enterics, and Candida spp. may also contribute to peri-implant infections (Slots and Rams 1991; Leonhardt et al. 1999).
In summary, orthopaedic and dental implants share a number of characteristics; failure of these implants is often due to development of a biofilm on the stem region, which blocks osseointegration and function.
Protection of Adherent Organisms in Biofilms
Bacterial colonization of orthopaedic and dental implants is complex, probably polymicrobial, and dependent on both host and bacterial factors. Within blood, synovial fluid, or saliva, bacteria exist as free-floating organisms or, more commonly, small clusters of bacteria (Fig. 1) (Malamud et al. 1984; Dastgheyb and Otto 2015).
Figure 1.
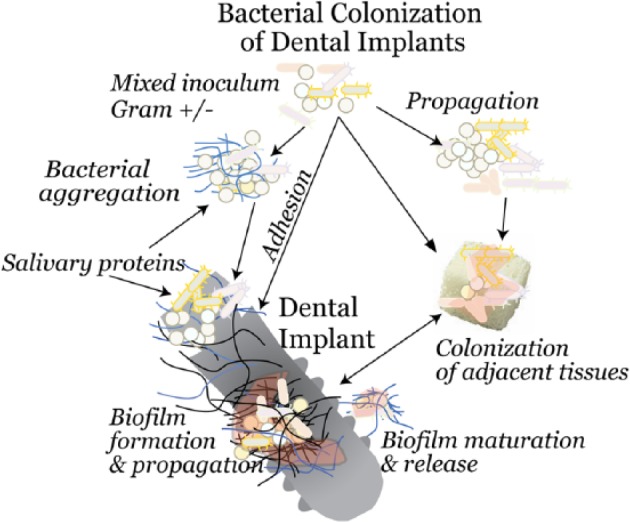
Stages in colonization of implants that interface with bone. Bacteria are always present in the oral environment. In a physiologic fluid, the organisms propagate, and some cluster together to form floating biofilms. Bacteria then either adhere to the protein-coated implants, with biofilm formation proceeding in a relatively protected niche, or localize to the bone around the implant. Nonadherent bacteria continue to be sloughed off from the surface-bound biofilms, seeding additional sites distant from the initial site of contamination.
The synovial fluid that bathes the joint surface is a proteoglycan-rich, viscous fluid that contains many proteins found in blood, with fibrinogen and fibronectin of special interest. These proteins, whether in synovial fluid or adsorbed onto the prosthesis surface, facilitate biofilm formation where both the floating and the adherent Staphylococcus aureus can exist as bacteria immobilized on a fibrous matrix encased in a biofilm polysaccharide slime (Dastgheyb et al. 2015).
Like in synovial fluid, bacterial agglutination can occur in the oral cavity through direct interaction with saliva or through adherence to the proteinaceous tooth pellicle, a thin film derived from saliva that is present even on a thoroughly cleaned tooth (Hojo et al. 2009). Specifically, oral Streptococci, such as Viridans streptococci and Streptococcus gordonii, can preferentially bind to proteins on the implant surface or in saliva, which include proline-rich glycoproteins, α-amylase, and proline-rich proteins. Interestingly, the proteins that promote clumping in synovial fluid (fibrinogen and fibronectin, among others) are inhibitory to saliva-mediated agglutination of oral pathogens (Malamud et al. 1984).
Whether in the oral or joint environment, these individual or clumped organisms preferentially adhere to an implant surface. Bacterial surface receptors and ionic interactions between charged groups on the bacterial membrane and side chains of the proteins(s) mediate this localization, where electrostatic interactions, including Lifshitz-Van der Waals forces, and acid-base bonding specifically contribute to adherence (Busscher et al. 2010). Once adherent, organisms evidence marked metabolic and phenotypic changes related to quorum sensing, further modified by the presence of other organisms populating the biofilm. In addition, the local oxygen tension, pH, and hydrodynamic/mechanical effects influence cell signaling, ion channel activity, osmotic relationships, and gene expression (Alsharif et al. 2015).
The adherent bacterial colony grows to create a 3-dimensional structure that some have compared to a tissue where the structure, composition, and microbial content vary from region to region (Costerton et al. 1999). For instance, bacteria that produce short-chain fatty acids in one region of an oral biofilm can serve as essential carbon sources for bacteria in other regions, establishing a symbiotic relationship (Hojo et al. 2009). Likewise, the architecture of the biofilm is porous, allowing fluid exchange and waste disposal. Since fluid flow varies from region to region, there are marked variations in pH within the biofilm (Costerton et al. 1999). As the biofilm matures, bacterial numbers increase in one region, which is offset by decreases in other regions due to a combination of indolence and cell death. Even the dying organisms contribute to the structure of the biofilm by depositing their DNA into the biofilm matrix (Otto 2013).
Once organisms are within the biofilm, there is a profound change in metabolism, which affects antibiotic susceptibility. Antibiotics tend to target functions of rapidly growing cells, such as cell wall synthesis (e.g., penicillins, cephalosporins, and vancomycin), protein synthesis (e.g., tetracyclines and aminoglycosides), and DNA replication (e.g., floxacins), all of which are significantly downregulated. While some antibiotics are more effective against biofilm bacteria than others, they all show reduced efficacy so that their minimal inhibitory concentration (MIC) can increase by as much as 1,000-fold (Otto 2013). It is important to emphasize that these bacteria are antibiotic recalcitrant rather than resistant. As the biofilm matures and bacteria slough off into the surrounding environment, antibiotic sensitivity is restored, similar to the parent planktonic bacteria (Otto 2013). As part of the indolence associated with biofilm bacteria, a subset of the bacteria is thought to transition to a persister phenotype. Persisters are bacteria that are recalcitrant to the effects of high antibiotic levels where persister formation occurs in a small percentage of bacteria within even planktonic cultures exposed to antibiotics. These persisters exist in an inactive state until antibiotic concentrations wane, when they emerge from quiescence. In this way, these cells can repopulate a depleted region or initiate biofilm formation. It has been suggested that the number of persisters is significantly higher in the biofilm state than in planktonic cultures (Lewis 2012).
Antibiotic-resistant bacteria can also form biofilms and show reduced antibiotic sensitivity. Importantly, these resistant bacteria, whether planktonic or localized to biofilms, are difficult to eradicate at antibiotic levels that are nontoxic. Unfortunately, within polymicrobial biofilms, conditions are ideal for acquisition of plasmids that carry antibiotic resistance genes.
One way to minimize bacterial colonization and biofilm formation is to create/engineer implants with surfaces that resist bacterial adherence and/or promote bacterial killing. The challenge is to generate surfaces that fulfill these criteria while remaining biocompatible and promoting osseointegration.
Responding to the challenges of biofilm infectivity, lack of healing, and its role as a nidus for infection, in vitro models have been developed to assess how specific organisms and/or new surfaces influence biofilm formation, the ecology of the system, mechanisms of bacterial adherence, and proliferation and sensitivity of biofilm organisms to antibiotics. Variables in the models include fluid flow, bathing medium, mono- or polymicrobial organisms, and substrate composition and placement. The reader is referred to excellent reviews by McBain (2009) and Coenye and Nelis (2010).
Implants with Antimicrobial Surfaces: Biofilm Formation and Osseointegration
To prevent biofilm formation on implants, 3 classes of antimicrobial surfaces have been created: 1) structured surfaces where topography, often at the nanoscale, decreases bacterial adhesion to retard establishment of infection; 2) surfaces that actively elute antimicrobials to avert bacterial adhesion and promote killing; and 3) surfaces containing permanently bonded agents that generate antimicrobial surfaces that prevent long-term bacterial adhesion.
Structured Surfaces
A variety of surfaces have been developed that decrease bacterial adhesion in vitro while being biocompatible. These structures include nanoparticle- and nanotube-modified surfaces, as well as engineered metal topographies and molecular structures. Common topographical modifications include alterations in charge, hydrophobicity, roughness, and porosity (Hasan and Chatterjee 2015). In 1 study, heat treatment and anodization of titanium (Ti) significantly reduced bacterial adhesion (1–2 logs). In addition, exposure to UV light caused a further reduction in bacterial counts (Del Curto et al. 2005). We would argue that this 1- to 2-log reduction in bacterial adhesion may be sufficient to prevent establishment of infection in cases where the inocula are low, such as happens during surgical procedures. Indeed, in 2 separate animal studies, we have found that this level of reduction was sufficient to prevent the establishment of infection (Antoci et al. 2007b; Stewart et al. 2012). However, in a more challenging situation with high bacterial inocula and/or decreased immune surveillance, in our opinion, these surfaces would likely be overwhelmed. Based on these findings, we would predict that a number of these surface modifications would retard establishment of infection, where some materials with such properties (i.e., the tantalum implants [Tokarski et al. 2015]) are already in clinical use.
Elution Systems
Relevant to both dental and orthopaedic therapy, antibiotic elution systems inhibit bacterial growth and, for the most part, prevent bacterial adhesion to the implant surface and adjacent tissues (Jepsen and Jepsen 2016). In many systems, supratherapeutic levels of antibiotics are only achieved within the first week (Humphrey et al. 1998; Bormann et al. 2014), with lower, continued elution for some time afterward (Fig. 2). These elution systems can therefore be problematic for the following reasons. First, an initial burst of antibiotic is often in the milligram range, which is then followed by an exponential decline. Depending on the antibiotic and the local environment (tissue fluid volume, pH, and circulation), initial quantities can be toxic to the already compromised bone (Antoci et al. 2007a). Second, when antibiotic concentration is high, a small percentage of bacteria will convert to a persister phenotype. When antibiotic levels wane, the persisters can regain their proliferative phenotype and, worse still, adhere to the now antibiotic-depleted delivery system (Lewis 2012). Finally, following the initial burst, the continued elution of antibiotics to subtherapeutic levels enhances selection of bacteria that exhibit a survival/growth advantage. This event can foster enrichment/emergence of antibiotic-resistant bacteria. It is important to note that despite these drawbacks and therapeutic implications, these systems, although imperfect, are used quite successfully to treat implant-associated infections (Da Rocha et al. 2015).
Figure 2.
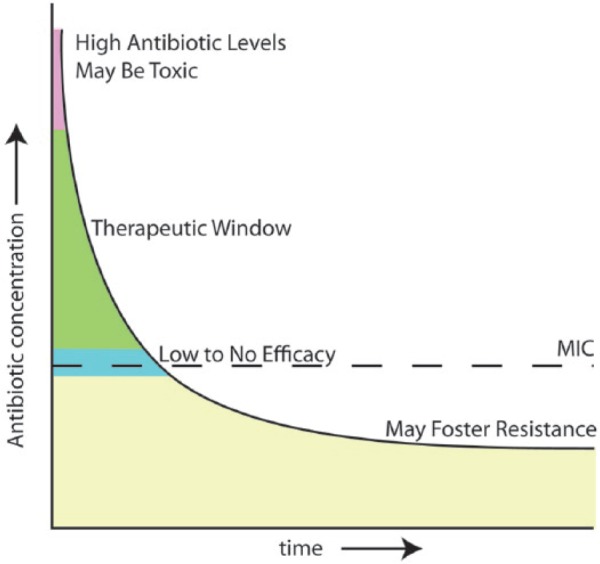
Elution profile of antibiotics in most controlled release systems. The therapeutic window usually has a rapid onset but may be maintained for up to a week. While antibiotic elution may continue for some time, antibiotic levels eventually fall below the minimal inhibitory concentration (MIC) and introduce the risk of allowing bacterial overgrowth in low to subinhibitory concentrations of antibiotics that may foster resistance.
In dentistry, elution methods developed to treat peri-implantitis include use of 1) antiseptics such as chlorhexidine digluconate, 2) minocycline spheres and tetracycline periodontal fibers, and 3) metals such as silver and copper (Jepsen and Jepsen 2016). When chlorhexidine was compared to minocycline spheres, minocycline was found to reduce pocket size (Renvert et al. 2004). In another study, the minocycline spheres did not offer an advantage over mechanical debridement, even after 12 mo (Bassetti et al. 2014). To date, results are inconclusive concerning the best local, chemical approach, and it is not clear if they offer an advantage over systemic antibiotic therapy and mechanical debridement (Javed et al. 2013).
Elution systems are commonly used to prevent infection associated with orthopaedic trauma and to treat periprosthetic joint infection; spinal infections tend to rely on systemic antibiotics (Kim et al. 2010). Following debridement of damaged tissue and aggressive lavage, gentamicin-eluting beads can be implanted into a contaminated trauma site to kill bacteria and allow placement of stabilization hardware. In the case of a periprosthetic joint infection, antibiotic (often tobramycin ± vancomycin) release from bone cement is used to eradicate organisms remaining in the joint and bone canal. Of course, once the antibiotic levels in either system drop below therapeutic levels, there is a concern that the depleted material may serve as a nidus for biofilm formation, as well as increasing the possibility for acquisition of resistant bacterial strains (Hinarejos et al. 2015).
A final system relies on the elution of silver and has been exploited for many years. The antimicrobial effects of silver are attributed to the production of Ag+ ions, which are toxic to bacterial cells, and this toxicity is probably related to the affinity of the silver for the bacterial cell membrane (Brennan et al. 2015). Silver elution/dissolution can occur over long time periods, where its antimicrobial activity may be enhanced by surface area and shape (Kumari et al. 2017). Silver-eluting orthopaedic and dental implants are being developed (Matsubara et al. 2015), although concerns about toxicity remain. This toxicity includes mitochondrial respiratory chain interruptions, interactions with sulfur-containing proteins, altered membrane permeability, and reactive oxygen species (ROS) production (McShan et al. 2014). Despite these problems, if other tests support sufficient biocompatibility and antibacterial activity, silver coatings may become attractive for controlling implant infection. As an aside, silver elution systems have been tested in urinary catheters in vitro and have been shown to be effective against coagulase-negative staphylococci (Thomas et al. 2015). However, despite impressive in vitro results, in clinical practice, outcomes in the urology field are mixed (Pickard et al. 2012).
Permanent Antimicrobial Surfaces
The third type of antibacterial system depends on tethering or cross-linking an antimicrobial molecule to the surface of the implant so that bacteria are killed upon adherence. These surfaces rely on the ability of the antimicrobial agent to retain its activity, even when covalently bonded to a surface. Tethered antimicrobial compounds include various chitosans and amines (Actis et al. 2013), antimicrobial peptides (de la Fuente-Nunez et al. 2016), and antibiotics (Hickok and Shapiro 2012).
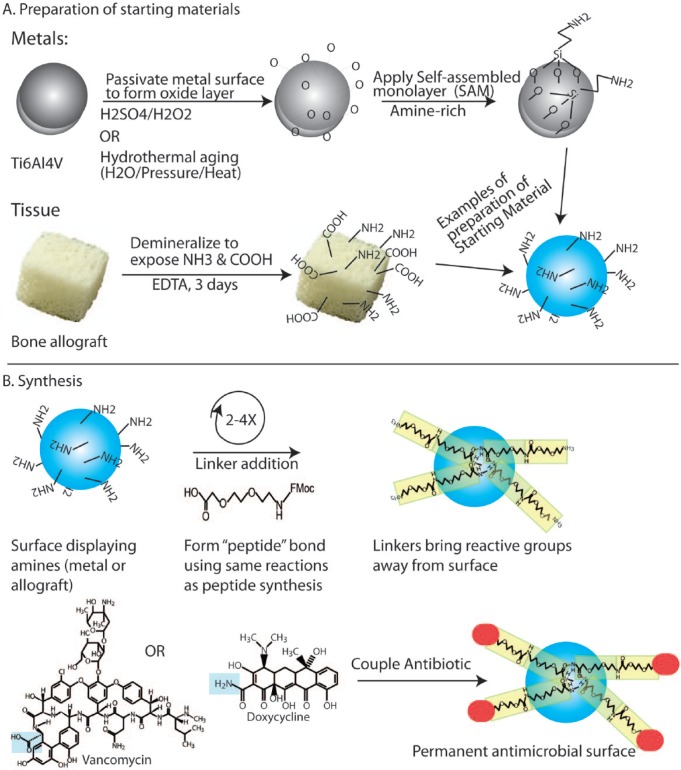
We and others have developed the chemistry to bond antibiotics to metal and other tissue surfaces (Danahy et al. 2004; Jose et al. 2005; Lawson et al. 2010). Implant metals, such as titanium, titanium alloy, cobalt chromium molybdenum alloys, and stainless steel, form metal oxides that are stable in the aqueous tissue environment. Antibiotics can be attached to these surfaces using a number of different linkages. Commonly, antibiotics are coupled to the metal via a sulfhydryl bond tethered to polydimethylsiloxane (PDMS) (Lim et al. 2013). Alternatively, despite their fragility, biodegradable hydrogels can be bound to metal surfaces; as their chemistry can be controlled to display and/or release antimicrobial agents, these hydrogels offer a nimble platform for combatting implant infection (de la Fuente-Nunez et al. 2016). Finally, our method of choice has been to covalently bond aminopropyltriethoxy silane (APTES) to the metal oxide, forming a self-assembled monolayer (SAM) (Lee et al. 2005), which bears a pendant primary amine. This amine can be used as a starting point for solid-state peptide synthesis to ultimately bond antibiotics such as vancomycin, tetracycline, and doxycycline (Fig. 3). In our hands, commercially pure titanium and titanium alloy (Ti6Al4V) SAMs with tethered vancomycin showed long-term (>1 y) stability under bench conditions (Antoci et al. 2008), whereas SAMs formed on stainless steel degraded over the course of ~2 to 3 wk.
Figure 3.
Synthetic steps in preparing antimicrobial surfaces. (A) Surface preparation of metal and tissues. Metal surfaces are passivated to create a fresh, abundant oxide layer. This oxide layer is allowed to couple with aminopropyltriethoxy silane (APTES), which will form a self-assembled monolayer on the surface of the metal. This silanization exposes primary amines, which are used for coupling of antibiotics. Similarly, tissues such as bone are partially demineralized, which allows retention of some mechanical properties while exposing more of the charged amino acids. In the synthesis here, these amines will be used to tether antibiotics. (B) Synthesis of permanent antimicrobial surfaces. Using the exposed amines, 2 to 4 aminoethoxyethoxy acetic acid (AEEA) linkers are sequentially coupled to bring the coupling site away from the surface. Antibiotics such as doxycycline or vancomycin are then coupled using reactions similar to those in peptide synthesis to form a covalently tethered antibiotic surface (Antoci, King, et al. 2007).
An important consideration is that antibiotics like vancomycin and members of the tetracycline family, including the glycylcyclines, reversibly bind to target groups within the bacterium. For example, vancomycin reversibly interacts with Lys-D-Ala-D-Ala peptides to block their incorporation into the peptidoglycan cell wall. We have shown that a vancomycin-titanium surface can be repeatedly challenged with bacteria and retain its biocidal activity (Antoci, Adams, et al. 2007). In contrast, a surface bearing the antibiotic gentamicin, an aminoglycoside, shows high biocidal activity that wanes when rechallenged. This loss of activity is presumably due to the antibiotic irreversibly binding to the bacterial ribosome (30S subunit). Because the binding is irreversible, the gentamicin surface becomes spent. In contrast, the vancomycin surface appears to release the bacterial fragments after lysis, presumably due to its reversibility (Antoci, Adams, et al. 2007).
In the design of the metal-antibiotic hybrid, it is important to consider the relationship of the antibiotic to the adherent organisms. Since vancomycin blocks events at the level of the cell wall, propinquity to its target could be achieved using a membrane-soluble linker extension. This extension would facilitate entrance of the antibiotic into the outer layers of the cell wall. In practice, we used 2 aminoethoxyethoxy acetic acid linkers (AEEA) to tether vancomycin, whereas the tetracycline family was tethered using 4 linkers (Hickok and Shapiro 2012). How the linked antibiotic kills the bacteria is still under investigation.
Like the surfaces that depend on topography for antimicrobial properties, the antibiotic-modified surfaces achieve a ~2- to 3-log reduction in colony-forming units (CFU) with a 105 CFU/mL inoculum of S. aureus. This degree of inhibition is dependent on the time and initial inoculum; lower inocula show greater percent reductions; longer times, unless the surface is overwhelmed, also show a trend toward sustained eradication (Ketonis et al. 2011). Others have modified either the synthetic procedures or induced vancomycin polymerization to slightly increase innate activity; they have found that both of these procedures result in increased antimicrobial efficacy (Danahy et al. 2004; Lawson et al. 2010).
Proteins derived from saliva, synovial fluid, or blood might mask antimicrobial activity associated with these surfaces. In our hands, serum coating of the vancomycin-titanium surfaces did not attenuate its efficacy (Antoci, King, et al. 2007), a result that was further confirmed by in vivo testing (Antoci et al. 2007b). Synovial fluid causes bacterial aggregation prior to adhesion to surfaces, and these preaggregated biofilms may be largely protected from permanent antimicrobial surfaces. In terms of saliva, both mixed and ducted saliva cause aggregation of bacteria and thus act in a similar manner to synovial fluid. In addition, proteins from mixed and ducted saliva adhere to the tooth surface, forming a pellicle, which also serves to aggregate oral bacteria. Accordingly, while existing models for testing antimicrobial activity against dental biofilms include simulated saliva, perhaps the only appropriate model is that which uses mixed saliva (Darrene and Cecile 2016). Measurement of the importance of any particular salivary component is confounded by the role of media, protein content, pH, electrolyte composition, and flow conditions, all of which can be varied depending on the in vitro biofilm model (McBain 2009). In summary, it is critical to assess true efficacy using appropriate physiological fluids in vitro and with in vivo models that test the appropriate physiological environment.
Properties of Antimicrobial Surfaces
Unlike the continuous elution associated with controlled-release systems, permanent surfaces are stable and only release antimicrobials when bonding is severed by mechanical or chemical means. The amount of antimicrobial linked to a permanent surface is very small (3 ng vancomycin/g Ti ([Antoci, Adams, et al. 2007]) or 1 vancomycin molecule/3.8 nm2 (Rottman et al. 2012), compared to the mg-g quantities associated with elution systems (Kuechle et al. 1991). Thus, for these permanent surfaces, the total concentrations due to breakdown or release are very low compared to that released if solid or liquid antimicrobials are used (Antoci, Adams, et al. 2007). The purposes of the 2 systems are thus distinct in that permanent surfaces do not attempt to treat the surrounding tissue but seek to remove the implant from the possibility of bacterial colonization and may be active over the long term; controlled-release systems treat the surrounding tissue while remaining uncolonized, but this can only be maintained during the limited period of therapeutic antibiotic elution.
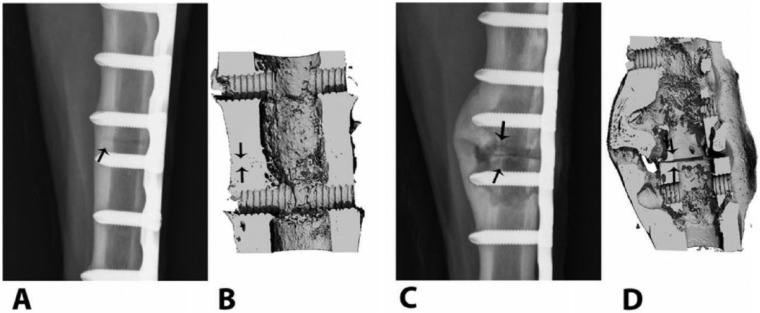
It is interesting to note that the surface-bound antibiotics appear to have extended stability compared to their solution half-life (Antoci et al. 2008). We have found that covalently linked vancomycin and tetracycline surfaces were stable on the shelf (in the dark) for many months and were active in the presence of a serum coating (Antoci et al. 2008; Davidson et al. 2015). Activity and, by implication, stability of the surface-bound antibiotics were tested over a 3-mo period using an infected sheep osteotomy model. Vancomycin-locking plates achieved complete bony union with no soft tissue infection, whereas control plates were grossly infected, with apparent nonunion (Fig. 4). Furthermore, osseointegration was apparent in all vancomycin-tethered implants (Stewart et al. 2012). Using antimicrobial peptides, the stability of the tethered antimicrobial layer has been confirmed by other workers (de la Fuente-Nunez et al. 2016). While these may have a shorter half-life due to protease sensitivity, this can be overcome by using protease-resistant peptidomimics. After surface tethering, these peptidomimics can achieve long-term antimicrobial stability and activity to offer advantages with respect to targeted antimicrobial activity and stability (de la Fuente-Nunez et al. 2016).
Figure 4.
Osteotomies repaired with vancomycin-tethered plates (A, B) and untreated, control plates (C, D). The representative craniocaudal digital radiograph of the site with the vancomycin-tethered plate made 90 d after osteotomy (A) is consistent with normal bone healing and callus formation at the osteotomy site (arrow), but the site with the control plate (C) shows progressive signs of cortical thinning, periosteal disruption, and osteolysis consistent with septic osteomyelitis at the osteotomy site (arrows). Three-dimensional reconstructed microcomputed tomography views of the same osteotomy sites in the vancomycin-tethered (B) and control animals (D) show persistence of the osteotomy gap (arrows) in the control, with a poorly organized lytic callus, enlarged medullary canal, and cortical thinning. Reproduced by permission from Stewart et al. (2012).
Mechanical stability can limit utility of permanent surfaces. The force associated with insertion of a screw into a bone, in our experiments, is sufficient to cause bond breakage (and thus loss of antibiotic coating) at areas of high force, such as screw threads. Thus, testing must account for the effects of push-pull forces on the tethered implant layer. Importantly, it is unknown whether the process of osseointegration of dental or orthopaedic prostheses will remove some of the tethered antibiotics. This possible mechanical stripping of the surface coating should be even greater for softer materials (hydrogels or polymers). However, the chemical bonding of antibiotics to an implant surface together with the acceptable mechanical stability suggests that this kind of implant would have major advantages for orthopaedic usage. Whether these types of surfaces would benefit dental implants, especially when there is an immune deficiency, needs further examination.
Finally, when permanent surfaces are used, questions will always be asked about antimicrobial resistance. As noted above, 1 molecule of vancomycin would be present per 3.8 nm2 of surface (Rottman et al. 2012). These quantities, if released, are insufficient to give a selective growth advantage to bacteria. Similarly, we have reasoned that the surface itself would not foster antibiotic resistance as the number of bacteria exposed to it is very small. We have tested whether methicillin-sensitive S. aureus would acquire vancomycin insensitivity in the presence of the permanent surface. To ensure sufficient exposure, loosely adherent S. aureus were exposed to the vancomycin-tethered surface for up to 3 mo. At no time did the sensitivity of the S. aureus to vancomycin change (Antoci, Adams, et al. 2007). However, if resistant bacteria were cultured in the presence of the surface, it is possible that they could populate the implant.
Conclusion and Future Directions
Currently, many new technologies are targeted for prevention of biofilm formation on implant surfaces. While the in vitro antibacterial effects appear to be promising, there is considerable variability in the test conditions employed, and therefore it is difficult to compare between trials. Thus, investigations using animal models that recapitulate the disease phenotype become even more critical. If permanent surfaces that show sustained in vitro efficacy can withstand the mechanical, chemical, and immunological assaults associated with an infected implant, it will be possible to significantly reduce the implant failures and associated bone loss that accompany peri-implantitis and orthopaedic infections.
Author Contributions
N.J. Hickok, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript; I.M. Shapiro, A.F. Chen, contributed to conception, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Keith Fitzgerald for his high-level technical support.
Footnotes
Research reported in this publication was supported by the US National Institutes of Health Award Number AR051303 (NJH), DE019901 (NJH), HD061053 (NJH, IMS), and T32 AR052273 (IMS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. NJH and IMS are Independence Blue Cross Investigators-in-Residence, and this work was also supported by the IBX-Jefferson Partnership.
NJH and IMS have intellectual property associated with part of this work. The authors declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Actis L, Gaviria L, Guda T, Ong JL. 2013. Antimicrobial surfaces for craniofacial implants: state of the art. J Korean Assoc Oral Maxillofac Surg. 39(2):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal VK, Bakhshi H, Ecker NU, Parvizi J, Gehrke T, Kendoff D. 2014. Organism profile in periprosthetic joint infection: pathogens differ at two arthroplasty infection referral centers in Europe and in the United States. J Knee Surg. 27(5):399–406. [DOI] [PubMed] [Google Scholar]
- Alsharif G, Ahmad S, Islam MS, Shah R, Busby SJ, Krachler AM. 2015. Host attachment and fluid shear are integrated into a mechanical signal regulating virulence in Escherichia coli O157:H7. Proc Natl Acad Sci U S A. 112(17):5503–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Implant Dentistry. 2017. Dental implants facts and figures. http://www.aaid.com/about/press_room/dental_implants_faq.html
- Antoci V, Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. 2007. a. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin Orthop Relat Res. 462:200–206. [DOI] [PubMed] [Google Scholar]
- Antoci V, Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. 2007. b. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin Orthop Relat Res. 461:88–95. [DOI] [PubMed] [Google Scholar]
- Antoci V, Jr, Adams CS, Parvizi J, Davidson HM, Composto RJ, Freeman TA, Wickstrom E, Ducheyne P, Jungkind D, Shapiro IM, et al. 2008. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials. 29(35):4684–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoci V, Jr, Adams CS, Parvizi J, Ducheyne P, Shapiro IM, Hickok NJ. 2007. Covalently attached vancomycin provides a nanoscale antibacterial surface. Clin Orthop Relat Res. 461:81–87. [DOI] [PubMed] [Google Scholar]
- Antoci V, Jr, King SB, Jose B, Parvizi J, Zeiger AR, Wickstrom E, Freeman TA, Composto RJ, Ducheyne P, Shapiro IM, et al. 2007. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 25(7):858–866. [DOI] [PubMed] [Google Scholar]
- Bassetti M, Schar D, Wicki B, Eick S, Ramseier CA, Arweiler NB, Sculean A, Salvi GE. 2014. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implan Res. 25(3):279–287. [DOI] [PubMed] [Google Scholar]
- Blus C, Szmukler-Moncler S, Khoury P, Orru G. 2015. Immediate implants placed in infected and noninfected sites after atraumatic tooth extraction and placement with ultrasonic bone surgery. Clin Implant Dent Rel Res. 17(Suppl 1):e287–e297. [DOI] [PubMed] [Google Scholar]
- Bormann N, Schwabe P, Smith MD, Wildemann B. 2014. Analysis of parameters influencing the release of antibiotics mixed with bone grafting material using a reliable mixing procedure. Bone. 59:162–172. [DOI] [PubMed] [Google Scholar]
- Brennan SA, Ni Fhoghlu C, Devitt BM, O’Mahony FJ, Brabazon D, Walsh A. 2015. Silver nanoparticles and their orthopaedic applications. Bone Joint J. 97B(5):582–589. [DOI] [PubMed] [Google Scholar]
- Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. 2010. Biofilm formation on dental restorative and implant materials. J Dent Res. 89(7):657–665. [DOI] [PubMed] [Google Scholar]
- Coenye T, Nelis HJ. 2010. In vitro and in vivo model systems to study microbial biofilm formation. J Microbiol Methods. 83(2):89–105. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science. 284(5418):1318–1322. [DOI] [PubMed] [Google Scholar]
- Crubzy E, Murail P, Girard L, Bernadou J-P. 1998. False teeth of the roman world. Nature. 391(6662):29. [DOI] [PubMed] [Google Scholar]
- Danahy MP, Avaltroni MJ, Midwood KS, Schwarzbauer JE, Schwartz J. 2004. Self-assembled monolayers of alpha,omega-diphosphonic acids on Ti enable complete or spatially controlled surface derivatization. Langmuir. 20(13):5333–5337. [DOI] [PubMed] [Google Scholar]
- Da Rocha HA, Silva CF, Santiago FL, Martins LG, Dias PC, De Magalhães D. 2015. Local drug delivery systems in the treatment of periodontitis: a literature review. J Int Acad Periodontol. 17(3):82–90. [PubMed] [Google Scholar]
- Darrene LN, Cecile B. 2016. Experimental models of oral biofilms developed on inert substrates: a review of the literature. Biomed Res Int. 2016:7461047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastgheyb S, Hammoud S, Ketonis C, Liu AY, Fitzgerald K, Parvizi J, Purtill J, Ciccotti M, Shapiro IM, Otto M, et al. 2015. Staphylococcal persistence due to biofilm formation in synovial fluid containing prophylactic cefazolin. Antimicrob Agents Chemother. 59(4):2122–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastgheyb SS, Otto M. 2015. Staphylococcal adaptation to diverse physiologic niches: an overview of transcriptomic and phenotypic changes in different biological environments. Future Microbiol. 10:1981–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H, Poon M, Saunders R, Shapiro IM, Hickok NJ, Adams CS. 2015. Tetracycline tethered to titanium inhibits colonization by Gram-negative bacteria. J Biomed Mater Res B Appl Biomater. 103(7):1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Nunez C, Cardoso MH, de Souza Candido E, Franco OL, Hancock RE. 2016. Synthetic antibiofilm peptides. Biochim Biophys Acta. 1858(5):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Curto B, Brunella MF, Giordano C, Pedeferri MP, Valtulina V, Visai L, Cigada A. 2005. Decreased bacterial adhesion to surface-treated titanium. Int J Artif Organs. 28(7):718–730. [DOI] [PubMed] [Google Scholar]
- Hasan J, Chatterjee K. 2015. Recent advances in engineering topography mediated antibacterial surfaces. Nanoscale. 7(38):15568–15575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok NJ, Shapiro IM. 2012. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 64(12):1165–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinarejos P, Guirro P, Puig-Verdie L, Torres-Claramunt R, Leal-Blanquet J, Sanchez-Soler J, Monllau JC. 2015. Use of antibiotic-loaded cement in total knee arthroplasty. World J Orthop. 6(11):877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo K, Nagaoka S, Ohshima T, Maeda N. 2009. Bacterial interactions in dental biofilm development. J Dent Res. 88(11):982–990. [DOI] [PubMed] [Google Scholar]
- Humphrey JS, Mehta S, Seaber AV, Vail TP. 1998. Pharmacokinetics of a degradable drug delivery system in bone. Clin Orthop Rel Res. (349):218–224. [DOI] [PubMed] [Google Scholar]
- Javed F, Alghamdi AS, Ahmed A, Mikami T, Ahmed HB, Tenenbaum HC. 2013. Clinical efficacy of antibiotics in the treatment of peri-implantitis. Int Dent J. 63(4):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen K, Jepsen S. 2016. Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000. 71(1):82–112. [DOI] [PubMed] [Google Scholar]
- Jose B, Antoci V, Jr, Zeiger AR, Wickstrom E, Hickok NJ. 2005. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem Biol. 12(9):1041–1048. [DOI] [PubMed] [Google Scholar]
- Ketonis C, Barr S, Adams CS, Shapiro IM, Parvizi J, Hickok NJ. 2011. Vancomycin bonded to bone grafts prevents bacterial colonization. Antimicrob Agents Chemother. 55(2):487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Suh KT, Kim SJ, Lee JS. 2010. Implant removal for the management of infection after instrumented spinal fusion. J Spinal Disord Tech. 23(4):258–265. [DOI] [PubMed] [Google Scholar]
- Kuechle DK, Landon GC, Musher DM, Noble PC. 1991. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin Orthop Rel Res. (264):302–308. [PubMed] [Google Scholar]
- Kumari M, Pandey S, Giri VP, Bhattacharya A, Shukla R, Mishra A, Nautiyal CS. 2017. Tailoring shape and size of biogenic silver nanoparticles to enhance antimicrobial efficacy against MDR bacteria. Microb Pathog. 105:346–355. [DOI] [PubMed] [Google Scholar]
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. 2010. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 468(1):52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MC, Hoth KC, Deforest CA, Bowman CN, Anseth KS. 2010. Inhibition of Staphylococcus epidermidis biofilms using polymerizable vancomycin derivatives. Clin Orthop Relat Res. 468(8):2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Brass DA, Morris R, Composto RJ, Ducheyne P. 2005. The effect of non-specific interactions on cellular adhesion using model surfaces. Biomaterials. 26(14):1721–1730. [DOI] [PubMed] [Google Scholar]
- Leonhardt Å, Renvert S, Dahlén G. 1999. Microbial findings at failing implants. Clin Oral Implants Res. 10(5):339–345. [DOI] [PubMed] [Google Scholar]
- Lewis K. 2012. Persister cells: molecular mechanisms related to antibiotic tolerance. Handb Exp Pharmacol. 2012(211):121–133. [DOI] [PubMed] [Google Scholar]
- Lim K, Chua RR, Saravanan R, Basu A, Mishra B, Tambyah PA, Ho B, Leong SS. 2013. Immobilization studies of an engineered arginine-tryptophan-rich peptide on a silicone surface with antimicrobial and antibiofilm activity. ACS Appl Mater Interfaces. 5(13):6412–6422. [DOI] [PubMed] [Google Scholar]
- Lindhe J, Meyle J; Group D of European Workshop on Periodontology. 2008. Peri-implant diseases: consensus report of the sixth European Workshop on Periodontology. J Clin Periodontol. 35(Suppl 8):282–285. [DOI] [PubMed] [Google Scholar]
- Malamud D, Brown C, Goldman R. 1984. Inhibition of bacterial aggregation by serum- and blood-derived proteins. Infect Immun. 43(1):386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, Jiranek WA, Berry DJ. 2015. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 97(17):1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara VH, Igai F, Tamaki R, Tortamano Neto P, Nakamae AE, Mori M. 2015. Use of silver nanoparticles reduces internal contamination of external hexagon implants by Candida albicans. Braz Dent J. 26(5):458–462. [DOI] [PubMed] [Google Scholar]
- McBain AJ. 2009. Chapter 4: in vitro biofilm models: an overview. Adv Appl Microbiol. 69:99–132. [DOI] [PubMed] [Google Scholar]
- McShan D, Ray PC, Yu H. 2014. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 22(1):116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. 2013. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med. 64:175–188. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Ghanem E, Azzam K, Davis E, Jaberi F, Hozack W. 2008. Periprosthetic infection: are current treatment strategies adequate? Acta Orthop Belg. 74(6):793–800. [PubMed] [Google Scholar]
- Pickard R, Lam T, Maclennan G, Starr K, Kilonzo M, McPherson G, Gillies K, McDonald A, Walton K, Buckley B, et al. 2012. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 16(47):1–197. [DOI] [PubMed] [Google Scholar]
- Powell CA, Mealey BL, Deas DE, McDonnell HT, Moritz AJ. 2005. Post-surgical infections: prevalence associated with various periodontal surgical procedures. J Periodontol. 76(3):329–333. [DOI] [PubMed] [Google Scholar]
- Renvert S, Lessem J, Lindahl C, Svensson M. 2004. Treatment of incipient peri-implant infections using topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement. J Int Acad Periodontol. 6(Suppl 4):154–159. [PubMed] [Google Scholar]
- Rottman M, Goldberg J, Hacking SA. 2012. Titanium-tethered vancomycin prevents resistance to rifampicin in Staphylococcus aureus in vitro. PLoS One. 7(12):e52883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. 2011. Understanding peri-implantitis: a strategic review. J Oral Implantol. 37(5):622–626. [DOI] [PubMed] [Google Scholar]
- Slots J, Rams TE. 1991. New views on periodontal microbiota in special patient categories. J Clin Periodontol. 18(6):411–420. [DOI] [PubMed] [Google Scholar]
- Stewart S, Barr S, Engiles J, Hickok NJ, Shapiro IM, Richardson DW, Parvizi J, Schaer TP. 2012. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 94(15):1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Soumya KR, Mathew J, Radhakrishnan EK. 2015. Inhibitory effect of silver nanoparticle fabricated urinary catheter on colonization efficiency of coagulase negative staphylococci. J Photochem Photobiol B. 149:68–77. [DOI] [PubMed] [Google Scholar]
- Tokarski AT, Novack TA, Parvizi J. 2015. Is tantalum protective against infection in revision total hip arthroplasty? Bone Joint J. 97B(1):45–49. [DOI] [PubMed] [Google Scholar]