Abstract
Aims:
To examine the association of patient-related factors with the effect of an in-hospital comprehensive geriatric assessment (CGA) on hip fracture mortality.
Methods:
Population-based, prospective data were collected on 1425 consecutive hip fracture patients aged ≥65 in a central hospital providing orthogeriatric service. Outcome was mortality at 1 month after hip fracture associated with receiving versus not receiving CGA.
Results:
Of the patients receiving CGA compared to those who did not, 8.5% versus12.0% had died within 1 month of the hip fracture (P = .028). In the age- and sex-adjusted Cox proportional hazards model, CGA was associated with a decreased risk of 1-month mortality in patients aged 80 to 89 years (hazard ratio [HR] 0.46, 95% confidence interval [CI]: 0.29-0.73), females (HR: 0.57, 95% CI: 0.38-0.86), having American Society of Anesthesiologists (ASA) score 1 to 3 (HR: 0.60, 95% CI: 0.37-0.99), taking 4 to 10 daily medications (HR: 0.59, 95% CI: 0.38-0.91), with a diagnosis of memory disorder (HR: 0.50, 95% CI: 0.29-0.88), with an estimated glomerular filtration rate <30 mL/min/1.73m2 (HR: 0.28, 95% CI: 0.10-0.76), or living in an assisted living accommodation (HR: 0.40, 95% CI: 0.21-0.76).
Conclusion:
Several modifiable and patient-related factors were associated with decreased risk of 1-month mortality when CGA was performed during hospitalization for hip fracture. Between “younger and fitter” and “oldest and frailest,” there is a large group of hip fracture patients whose survival can be improved by in-hospital CGA.
Keywords: hip fracture, orthogeriatric care, comanaged care, comprehensive geriatric assessment, mortality
Introduction
Geriatric hip fractures are an increasing burden as the world population ages. The consequences of hip fractures include high mortality,1-3 long-term disabilities1,4 and decreased quality of life,1 and high costs of care.3
According to a consensus statement, geriatricians may provide the greatest benefit when caring for the most vulnerable older adults,5 such as those with hip fracture. To compliment surgical care, various models of multidisciplinary care for patients have been developed.6 Current literature suggests the use of a comprehensive care approach to decrease mortality in a randomized setting,7 in a dedicated hip fracture unit8 and in register-based studies.9 On the other hand, implementation of a clinical pathway with a standardized set of orders10 or including an inpatient geriatric consultation team in the care model11 demonstrated no effect on mortality. A protocol-driven comanaged comprehensive care system combining a clinical pathway and geriatric care also reduces mortality12 but not without the involvement of a geriatrician.13
The implementation and components of orthogeriatric care models vary,6,10 making it challenging to compare different models. The background components of effective orthogeriatric care are not very well understood or researched.14 In addition, older hip fracture patients are a heterogeneous group15,16 and the beneficial actions of care and rehabilitation requirements may vary, which further complicates studies of optimal orthogeriatric models.
Moving forward, given the increasing number of hip fractures, limited health-care resources, and the short supply of geriatricians, there is a pressing need to clarify what truly is effective orthogeriatric care. In order to increase equity and effectiveness, targeted and tailored services may be needed. We examined the association of patient-related factors with the effect of an in-hospital comprehensive geriatric assessment (CGA; Table 1) on 1-month mortality among older hip fracture patients in an orthopedic ward during implementation of orthogeriatric hip fracture program (HFP).
Table 1.
Summary of Current Components of Seinäjoki Central Hospital Hip Fracture Program (HFP).
| Components For All Patients | Components of comprehensive geriatric assessment (CGA; when available) |
|---|---|
Standardized and detailed set of orders on
Preround interview by a geriatric hip fracture nurse
Discharge Criteria
|
Interdisciplinary orthogeriatric ward rounds on weekdays
Instructions and suggestions to discharge destination
|
Methods
Study Population
This is a retrospectively analyzed study of prospectively collected, population-based data on 1445 consecutive hip fracture patients aged ≥ 65 years having their first hip fracture between September 2007 and August 2014. The final study population comprised 1425 hip fracture patients as 20 (1.4%) patients declined participation in the study. Pathologic and periprosthetic fractures were excluded. The data were collected at Seinäjoki Central Hospital, Finland, which is the only hospital that provides acute surgical care in the Hospital District of Southern Ostrobothnia, which has a population of 199 000.
Hip Fracture Program and Study Design
Hip fracture program was first initiated in our hospital in 2007 with the goal of improving the care of hip fracture patients in accordance with evidence-based guidelines. First, a database with demographic, medical, surgical, functional, social, and outcome measures was established. Geriatrician-led interdisciplinary rounds began in 2008. A multidisciplinary orthogeriatric committee was established in 2009 and includes physicians from geriatrics, anesthesia, and orthopedic surgery; nurses from the orthopedic ward; and physiotherapists. Other experts are consulted if needed. The first written HFP with a standardized set of orders for hip fracture patients’ hospital stay was delivered in 2009. The integrated care model is of shared care: Patients are within an orthopedic ward, but the responsibility for the care of the patient is shared between the orthopedic surgeon and the geriatrician. The orthopedic surgeon sees the patient daily and the geriatrician on weekdays, and both services write their own orders. Resident physicians provide some of the care in this model as well.
Since its initiation, the HFP has widely expanded and is regularly updated by the orthogeriatric committee, and it has been stabilized from a project to a permanent model of care. The 2013 update of the HFP includes extensive instructions on pre-, peri-, postoperative, and surgical care and CGA, discharge criteria, and recommendations for postdischarge care (Table 1). The emphasis is on detailed, individually adjusted, and multidisciplinary care throughout and after the hospitalization. The physicians and nurses are encouraged to focus on the HFP through continuous education. A dedicated orthogeriatric nurse coordinates the service in the orthopedic ward.
The HFP was developed and implemented in a real-life setting with minimal additional resources. There are only a few posts for geriatricians in our hospital, and, like in many regions in Finland, occasional shortage of geriatricians has occurred. As a consequence of this, CGA has not been performed at all times. However, when a geriatrician is available, every hip fracture patient in the orthopedic ward receives CGA without any patient selection by exclusion or inclusion criteria. In the present study, we compared the effect of receiving CGA versus not receiving CGA while hospitalized for hip fracture on 1-month mortality during implementation of the HFP. To identify the specific patient-related factors affecting mortality when combined with CGA, we examined the association of baseline characteristics with the effect of CGA on mortality. By CGA, we mean the components of care as presented in Table 1.
Data Collection
During hospitalization, the patients’ medical records and interview conducted by a nurse with the patient or a caregiver were used. Data were collected on age, sex, fracture type, American Society of Anesthesiology (ASA) score, on-admission serum creatinine, number of regularly taken medications, regular or as-needed use of hypnotic benzodiazepines and z-hypnotics (BZD-Z; midazolam, temazepam, nitrazepam, triazolam, zaleplon, zolpidem, and zopiclon), prefracture diagnosis of memory disorder, prefracture mobility level and living arrangements, and receiving or not receiving CGA. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate estimated glomerular filtration rate (eGFR). The results were categorized into 4 groups: ≥ 60 mL/min/1.73 m2 (normal to mildly decreased eGFRCKD-EPI), 45 to 59 mL/min/1.73 m2 (mildly to moderately decreased eGFRCKD-EPI), 30 to 44 mL/min/1.73 m2 (moderately to severely decreased eGFRCKD-EPI), and under 30 mL/min/1.73 m2 (severely decreased eGFRCKD-EPI or kidney failure). The dates of death were provided by the National Population Register Center and extracted from the electronic patient files of the hospital. There were no losses to mortality follow-up.
Statistical Analyses
The distribution of patient-related factors in case numbers and percentages according to the CGA were calculated. Differences were tested using Pearson χ2 test or Fisher exact test (Table 2). One-month (1-30 days from hip fracture) mortality was analyzed by age- and sex-adjusted Cox proportional hazards models (Table 3). Age- and sex-adjusted association of receiving versus not receiving CGA with mortality separately in each group of the patient-related factors were performed using the Cox proportional hazards model showing results by hazard ratios with 95% confidence intervals (Table 4). One-month mortality was illustrated by a survival curve (Figure 1). A P value of <.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS for Windows, version 23.0 (IBM Corp, Armonk, New York).
Table 2.
Distribution of the Patient-Related Factors and Outcome Variables According to Comprehensive Geriatric Assessment.a,b
| Patient-related factors | Total | Comprehensive geriatric assessment | P Value | |
|---|---|---|---|---|
| N = 1425 | Yes (n=886) | No (n=539) | ||
| Age, n (%) | .268 | |||
| 65-79 | 410 (29) | 247 (28) | 163 (30) | |
| 80-89 | 759 (53) | 469 (53) | 290 (54) | |
| 90 or over | 256 (18) | 170 (19) | 86 (16) | |
| Sex, n (%) | .707 | |||
| Women | 1062 (75) | 657 (74) | 405 (75) | |
| Men | 363 (26) | 229 (26) | 134 (25) | |
| ASA score, n (%) | .002 | |||
| 1-3 | 1047 (74) | 672 (76) | 375 (70) | |
| 4-5 | 354 (25) | 206 (23) | 148 (28) | |
| Number of regularly taken medications, n (%) | .097 | |||
| < 4 | 247 (17) | 154 (17) | 93 (17) | |
| 4-10 | 916 (64) | 554 (63) | 362 (67) | |
| > 10 | 261 (18) | 177 (20) | 84 (16) | |
| BZD-Z | .153 | |||
| No | 1033 (73) | 655 (74) | 378 (70) | |
| Yes | 391 (27) | 230 (26) | 161 (30) | |
| Diagnosis of memory disorder, n (%) | .272 | |||
| No | 1038 (73) | 635 (72) | 403 (75) | |
| Yes | 379 (27) | 247 (28) | 132 (25) | |
| eGFRCKD-EPI, n (%) | .167 | |||
| > 60 mL/min/1.73m2 | 821 (58) | 490 (55) | 331 (61) | |
| 45-59 mL/min/1.73m2 | 287 (29) | 194 (22) | 93 (17) | |
| 30-44 mL/min/1.73m2 | 193 (14) | 125 (14) | 68 (13) | |
| < 30 mL/min/1.73m2 | 87 (6) | 55 (6) | 32 (6) | |
| Mobility level, n (%) | .070 | |||
| Outdoors unassisted | 743 (52) | 467 (53) | 276 (51) | |
| Indoors unassisted | 557 (41) | 354 (40) | 223 (41) | |
| Assisted only | 70 (5) | 46 (5) | 24 (5) | |
| Unable to walk | 25 (2) | 17 (2) | 8 (2) | |
| Living arrangements, n (%) | .002 | |||
| Home | 565 (40) | 362 (41) | 203 (38) | |
| Home with organized homecare | 399 (28) | 235 (27) | 164 (30) | |
| Assisted living accommodation | 237 (17) | 136 (15) | 101 (19) | |
| Institutionalized | 213 (15) | 150 (17) | 62 (12) | |
| Fracture type, n (%) | .545 | |||
| Neck of femur | 886 (62) | 541 (61) | 345 (64) | |
| Intertrochanteric | 458 (32) | 292 (33) | 166 (31) | |
| Subtrochanteric | 80 (6) | 52 (6) | 28 (5) | |
Abbreviations: ASA, American Society of Anesthesiologists; BZD-Z, hypnotic benzodiazepines and z-hypnotics (midazolam, temazepam, nitrazepam, triazolam, zaleplon, zolpidem, and zopiclon); eGFRCKD-EPI, estimated glomerular filtration rate calculated by the Chronic Kidney Disease Epidemiology Collaboration equation.
an = 1425.
bMissing values are not shown but were tested and included in the percentages. Differences between groups were tested by Pearson χ2 test or Fisher exact test. Statistically significant P values (P < .05) are bolded.
Table 3.
Distribution and Associations of the Patient-Related Factors and Comprehensive Geriatric Assessment According to 1-month Mortality.a
| Patient-related factors | 1-Month Mortality | ||
|---|---|---|---|
| Alive, n=1285 | Deceased, n=140 | Age- and Sex Adjusted | |
| n (%) | n (%) | HR (95% CI) | |
| Age | |||
| 65-79 | 389 (30) | 21 (15) | 1.00 |
| 80-89 | 683 (53) | 76 (54) | 2.21 (1.36-3.59) |
| 90 or over | 213 (17) | 43 (31) | 3.87 (2.28-6.55) |
| Sex | |||
| Women | 970 (76) | 92 (66) | 1.00 |
| Men | 315 (25) | 48 (34) | 1.80 (1.26-2.45) |
| ASA score | |||
| 1-3 | 983 (77) | 64 (46) | 1.00 |
| 4-5 | 290 (23) | 64 (46) | 2.75 (1.94-3.91) |
| Number of regularly taken medications | |||
| < 4 | 236 (18) | 11 (8) | 1.00 |
| 4-10 | 832 (65) | 84 (60) | 1.94 (1.03-3.63) |
| > 10 | 217 (17) | 44 (32) | 3.67 (1.89-7.12) |
| BZD-Z | |||
| No | 937 (73) | 96 (69) | 1.00 |
| Yes | 348 (27) | 43 (31) | 1.09 (0.76 -1.56) |
| Diagnosis of memory disorder | |||
| No | 952 (74) | 86 (61) | 1.00 |
| Yes | 330 (26) | 49 (35) | 1.55 (1.09-2.20) |
| eGFRCKD-EPI | |||
| > 60 mL/min/1.73m2 | 766 (60) | 55 (39) | 1.00 |
| 45-59 mL/min/1.73m2 | 252 (20) | 35 (25) | 1.69 (1.10-2.59) |
| 30-44 mL/min/1.73m2 | 164 (13) | 29 (21) | 1.93 (1.22-3.07) |
| < 30 mL/min/1.73m2 | 69 (5) | 18 (13) | 2.99 (1.75-5.12) |
| Mobility level | |||
| Outdoors unassisted | 718 (56) | 25 (18) | 1.00 |
| Indoors unassisted | 486 (38) | 91 (65) | 4.63 (2.95-7.28) |
| Assisted only | 58 (5) | 12 (9) | 4.77 (2.38-9.59) |
| Unable to walk | 21 (2) | 4 (3) | 4.04 (1.40-11.7) |
| Living arrangements | |||
| Home | 543 (42) | 22 (16) | 1.00 |
| Home with organized homecare | 369 (29) | 30 (21) | 1.79 (1.02-3.14) |
| Assisted living accommodation | 197 (15) | 40 (29) | 3.97 (2.31-6.81) |
| Institutionalized | 169 (13) | 44 (31) | 5.08 (2.98-8.65) |
| Fracture type | |||
| Neck of femur | 800 (62) | 86 (61) | 1.00 |
| Intertrochanteric | 410 (32) | 48 (34) | 0.99 (0.69 -1.41) |
| Subtrochanteric | 74 (6) | 6 (4) | 0.65 (0.28 -1.49) |
| Comprehensive Geriatric Assessment | |||
| No | 474 (37) | 65 (46) | 1.00 |
| Yes | 811 (63) | 75 (54) | 0.63 (0.45-0.87) |
Abbreviations: ASA, American Society of Anesthesiologists, BZD-Z, hypnotic benzodiazepines and z-hypnotics (midazolam, temazepam, nitrazepam, triazolam, zaleplon, zolpidem, and zopiclon), CI, confidence interval; eGFRCKD-EPI, estimated glomerular filtration rate calculated by the Chronic Kidney Disease Epidemiology Collaboration equation; HR, hazard ratio.
an = 1425.
bMissing values are not shown but were tested and included in the percentages. Associations with mortality were tested by Cox hazard regression models showing results by HRs and 95% CIs.
Table 4.
Age- and Sex-Adjusted Effect of In-hospital CGA (Total n = 1425, CGA n = 886, no CGA n = 539) on Mortality 1 Month After Hip Fracture in the Groups of Patient-Related Factors.a
| Mortality Ratio at 1 Month Comparing Groups of CGA versus non-CGA (Total Deaths n=140) | ||||
|---|---|---|---|---|
| Patient-related factors | Total (N) | Deaths, n (%) | HR | 95% CI |
| Age | ||||
| 65-79 | 410 | 21 (5.1) | 0.74 | 0.31-1.75 |
| 80-89 | 759 | 76 (10.0) | 0.46 | 0.29-0.73 |
| 90 or over | 256 | 43 (16.8) | 0.98 | 0.52-1.85 |
| Sex | ||||
| Women | 1062 | 92 (8.7) | 0.57 | 0.38-0.86 |
| Men | 363 | 48 (13.2) | 0.76 | 0.42-1.34 |
| ASA | ||||
| 1-3 | 1047 | 64 (6.1) | 0.60 | 0.37-0.99 |
| 4-5 | 354 | 64 (18.1) | 0.86 | 0.53-1.42 |
| Number of regularly taken medications | ||||
| < 4 | 247 | 11 (4.5) | 0.58 | 0.17-1.96 |
| 4-10 | 916 | 84 (9.2) | 0.59 | 0.38-0.91 |
| > 10 | 261 | 44 (16.9) | 0.58 | 0.32-1.06 |
| BZD-Z | ||||
| No | 1033 | 97 (9.4) | 0.75 | 0.50-1.13 |
| Yes | 391 | 43 (11.0) | 0.38 | 0.21-0.73 |
| Diagnosis of memory disorder | ||||
| No | 1038 | 86 (8.3) | 0.70 | 0.46-1.07 |
| Yes | 379 | 49 (12.9) | 0.50 | 0.29-0.88 |
| eGFRCKD-EPI | ||||
| > 60 mL/min/1.73m2 | 821 | 55 (6.7) | 0.73 | 0.43-1.24 |
| 45-59 mL/min/1.73m2 | 287 | 35 (12.2) | 0.46 | 0.24-0.90 |
| 30-44 mL/min/1.73m2 | 193 | 29 (15.0) | 0.79 | 0.36-1.72 |
| < 30 mL/min/1.73m2 | 87 | 18 (20.7) | 0.28 | 0.10-0.76 |
| Mobility level | ||||
| Outdoors unassisted | 743 | 25 (3.4) | 0.52 | 0.24-1.14 |
| Indoors unassisted | 557 | 91 (16.3) | 0.75 | 0.49-1.14 |
| Assisted only | 70 | 12 (17.1) | 0.77 | 0.24-2.42 |
| Unable to walk | 25 | 4 (16.0) | 2.32 | 0.10-53.4 |
| Living arrangements | ||||
| Home | 565 | 22 (3.9) | 0.75 | 0.32-1.75 |
| Home with organized homecare | 399 | 30 (7.5) | 0.81 | 0.39-1.69 |
| Assisted living accommodation | 237 | 40 (16.9) | 0.40 | 0.21-0.76 |
| Institutionalized | 213 | 44 (20.7) | 0.73 | 0.39-1.37 |
| Fracture type | ||||
| Neck of femur | 886 | 86 (9.7) | 0.44 | 0.29-0.68 |
| Intertrochanteric | 458 | 48 (10.5) | 1.20 | 0.65-2.21 |
| Subtrochanteric | 80 | 6 (7.5) | 0.50 | 0.10-2.48 |
Abbreviations: ASA, American Society of Anesthesiologists, BMI, body mass index; BZD-Z, hypnotic benzodiazepines and z-hypnotics (midazolam, temazepam, nitrazepam, triazolam, zaleplon, zolpidem, and zopiclon), eGFRCKD-EPI, estimated glomerular filtration rate calculated by the Chronic Kidney Disease Epidemiology Collaboration equation.
aStatistically significant P values (P < .10) are bolded.
Figure 1.
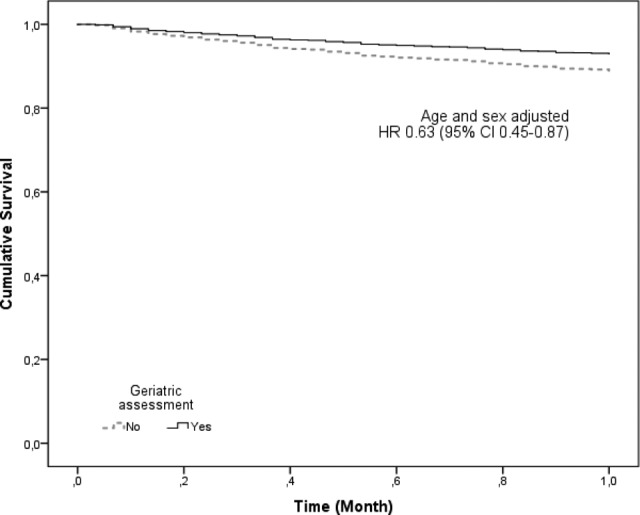
One-month survival after hip fracture according to comprehensive geriatric assessment by age- and sex-adjusted Cox proportional hazard model.
Ethical Consideration
The study was performed according to the 1964 Helsinki declaration and its later amendments and approved by the Ethics Committee of the Hospital District of South Ostrobothnia. Written informed consent was obtained from the participants or their caregivers.
Results
Data on 1425 hip fracture patients were available (Tables 2 and 3). The median age was 84 years (interquartile range: 78-88, range: 65-104), and the median length of stay was 6 days (interquartile range: 5-7, range: 1-37). Of the 1425 patients, 886 (62%) received CGA during hospitalization. Of the patients, 36 (3%) patients died during acute hospitalization and 140 (10%) patients within 1 month. Of the patients receiving CGA, 8.5% died within 1 month of the hip fracture, compared to 12.0% of the patients not receiving CGA (P = .028).
Comprehensive Geriatric Assessment
The patients receiving CGA while hospitalized, compared to those who did not, more likely had an ASA score of 1 to 3 (P = .002) and lived at home or in an institution (P = .002). Age, sex, number of regularly taken medications, use of BZD-Zs, diagnosis of memory disease, eGFRCKD-EPI, prefracture mobility level, or fracture type were not significantly different between the 2 groups (Table 2).
Age- and Sex-Adjusted 1-Month Mortality
In the age- and sex-adjusted Cox regression analysis, patients with older age, higher ASA score, higher number of medications in regular use, having a diagnosis of memory disease, lower eGFRCKD-EPI, living in more supported living accommodations, having lower mobility class, and male sex had a greater likelihood of dying within 1 month of the hip fracture (Table 3). The patients receiving CGA were significantly more likely to survive at 1 month after the hip fracture than those not receiving CGA (Table 3). The survival curve is shown in Figure 1.
Age- and Sex-Adjusted Analyses of the Effect of CGA With Mortality in the Groups of Patient-Related Factors
In the age- and sex-adjusted Cox proportional hazards model, CGA was significantly associated with decreased risk of 1-month mortality in patients aged 80 to 89 years, female sex, ASA score 1 to 3, using regular or as-needed BZD-Zs, having a diagnosis of memory disease, taking 4 to 10 medications daily, having eGFRCKD-EPI 45 to 59 mL/min/1.73, m2 or < 30 mL/min/1.73 m2, living in an assisted-living accommodation, or having the fracture in the neck of the femur (Table 4).
Discussion
Based on our findings, receiving CGA while hospitalized for hip fracture was associated with decreased 1-month mortality. This corroborates with previous literature of HFPs having a protective impact on short-term mortality7-9,12 Moreover, our study revealed several potentially medically modifiable factors and factors related to prefracture patient characteristics that could explain the protective effect of in-hospital CGA on short-term mortality.
In this study, patients aged 80 to 89 years benefitted from the CGA in relation to mortality, whereas younger or older patients did not. Older age is a well-known risk factor for mortality after hip fracture.2,17 Younger hip fracture patients are less frail15 and more likely to survive the hip fracture regardless of CGA. Nonagenarians with hip fracture are high-risk patients and are more often anemic and have more adverse events while hospitalized.17 In our study, CGA was associated with decreased mortality in women but not in men. Although women suffering hip fracture are older,18 male hip fracture patients have more chronic comorbidities,18 more severe health conditions, and a higher risk of complications during hospitalization for hip fracture.19 In addition, male sex itself is a risk factor for mortality in this patient group.2 It might be that in women, the conditions leading to hip fracture that are more of a concern are general frailty, including many potentially modifiable factors, in which case CGA can be more valuable in relation to mortality. The findings also imply that older men require specific attention regarding hip fracture care. According to the ASA classification, the general health of patients with a score of 1 to 3 varies from healthy to having severe systemic disease.20 Based on our results, the survival potential of these patients was better when CGA was performed.
An important part of the CGA is careful and critical evaluation of the patients’ medications. Our findings revealed that patients taking 4 to 10 regular medications or using BDZ-Z benefitted from CGA in relation to mortality. In a recent study by Gosch et al, only 9.6% of the hip fracture patients were taking appropriate medications.21 High number of medications22 and use of BZDs23 are associated with chronic illnesses, which increase the importance of input from a geriatrician. Renal insufficiency, such as multiple medication use, might be one of the few modifiable risk factors, as the patients with even severe renal insufficiency (eGFRCKD-EPI < 30 mL/min/1.73m2) on admission showed improved survival if assessed by a geriatrician. This might be partly due to a synergistic effect with a medication evaluation because the prevalence of potentially inappropriate medications in older patients with chronic kidney disease is high.24 The protective association of CGA in patients with renal dysfunction may also be explained by more careful fluid therapy. Dehydration is common among hip fracture patients and may increase the risk of acute renal dysfunction with a potentially poor prognosis.25
During the HFP, CGA was associated with decreased mortality among patients with a prefracture-diagnosed memory disorder. Dementia is a well-known risk factor for mortality among hip fracture patients.2 Orthogeriatric programs not excluding cognitively impaired patients have been successful in reducing in-hospital7 and 1-year8 mortality. In a subgroup analysis of hip fracture patients with dementia, the intervention group experienced fewer complications and better functional recovery, but the multidisciplinary intervention program had no effect on mortality.26 Poor knowledge of the engagement and recovery capacity of patients with dementia affects their access to rehabilitation after hip fracture,27 and this may also impact acute care decisions. A geriatric approach includes individual consideration for each patient and no denial of treatment or rehabilitation based merely on a diagnosis of dementia. Furthermore, dementia is an independent risk factor for iatrogenic conditions such as delirium28 in hip fracture patients. An optimistic attitude combined with professional care, including delirium prevention,6 may explain some of the results of our study.
Patients living in assisted-living accommodations are too frail to survive at home but well enough to avoid institutional living. In our study, this patient group benefitted greatly from CGA with regard to mortality. Community-dwelling hip fracture patients are generally younger and fitter29 and therefore may have a better prognosis regardless of the geriatrician’s input. Patients living in long-term residential care are generally in worse health and are thus more likely to have a poorer outcome,30 and the geriatricians’ principal role is to ensure quality of care toward the end of life.
In the present study, CGA was associated with improved short-time survival when the patients had a femoral neck fracture. Patients experiencing other fracture types are older,17,31 have more comorbidities,31 and a higher risk of mortality2,31 which may determine the prognosis beyond the effects of CGA.
One of the main strengths of the study is the prospective and population-based design. Also, the data were collected systematically and almost entirely by 1 individual. In addition, cognitive impairment and institutional living were not exclusion criteria, which increases the generalizability of the study. The study also has limitations. Due to the observational and noninterventional nature of this real-life study, there may be some uncertainty in the results. Toward the end of the study time, HFP has become more comprehensive and staff more experienced. Furthermore, there has been secular change toward overall increase in the awareness of the needs of this specific population. Also, although being otherwise similar, the groups receiving versus not receiving CGA differed significantly by the ASA scores and living arrangements. This is due to the fact that the groups were not predetermined or counterbalanced but were formed based on day-to-day availability of a geriatrician and without preselection of patients. Further studies are warranted to examine whether the findings observed in the present study also apply to other outcomes such as readmissions, mobility and living arrangements, and on mortality in longer term. We believe that a somewhat longer centralized acute postoperative period and rehabilitation in a dedicated orthogeriatric unit with in-hospital CGA is needed to further improve the outcomes.
In conclusion, the observation in our study of the protective association of in-hospital CGA in patients with multiple medications and renal insufficiency with hip fracture mortality highlights the significance of comprehensive and proactive medical assessment and interventions as fundamental part of acute orthogeriatric care. The orthogeriatric approach for younger and fitter patients did not have an effect on short-time mortality. Also, in relation to mortality, the oldest and frailest patients with the worst prognosis did not benefit of it either. These patients are, however, at the core of geriatric know-how,5 and the quality of care for those patients that are toward the end of life should be improved by having a geriatrician in the hip fracture team. After all, HFPs aim not only at reducing mortality but also at improving the quality of care.10,12 Between the extremes of low-risk and high-risk patients is a large group of patients whose potential to survive might go unnoticed in traditional care. Including a geriatrician and a CGA in the HFP can actually save the lives of these patients.
Acknowledgments
We would like to acknowledge Kaisu Haanpää, RN for her expert data collection.
Authors’ Note: The data sets supporting the conclusions of this article are not available in an open access repository because the data sets contain information by which the participants may be directly or indirectly identified. Informed consent from participants and the approval by the Ethics Committee of the Hospital District of South Ostrobothnia was obtained for publication of study results, but not for the publication of patient raw-data separately. Further, the data used in this study are a part of a clinical quality register of Seinäjoki Central Hospital and is continuously being updated. If anyone is interested in exploring specific issue, please contact Maria S. Nuotio, MD, PhD. Email: maria.nuotio@epshp.fi. Tel. +358-6-41533179.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the Competitive Research Fund of the Hospital District of Southern Ostrobothnia; and by the Competitive State Research Financing of Seinäjoki Central Hospital. The institutions of financial support did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
ORCID iD: Hanna M. Pajulammi, MD http://orcid.org/0000-0003-4249-1672
References
- 1. Griffin XL, Parsons N, Achten J, Fernandez M, Costa ML. Recovery of health-related quality of life in a United Kingdom hip fracture population. the Warwick hip trauma evaluation--a prospective cohort study. Bone Joint J. 2015;97-B(3):372–382. [DOI] [PubMed] [Google Scholar]
- 2. Hu F, Jiang C, Shen J, Tang P, Wang Y. Preoperative predictors for mortality following hip fracture surgery: a systematic review and meta-analysis. Injury. 2012;43(6):676–685. [DOI] [PubMed] [Google Scholar]
- 3. Nikitovic M, Wodchis WP, Krahn MD, Cadarette SM. Direct health-care costs attributed to hip fractures among seniors: a matched cohort study. Osteoporos Int. 2013;24(2):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertram M, Norman R, Kemp L, Vos T. Review of the long-term disability associated with hip fractures. Inj Prev. 2011;17(6):365–370. [DOI] [PubMed] [Google Scholar]
- 5. Warshaw GA, Bragg EJ, Fried LP, Hall WJ. Which patients benefit the most from a geriatrician’s care? consensus among directors of geriatrics academic programs. J Am Geriatr Soc. 2008;56(10):1796–1801. [DOI] [PubMed] [Google Scholar]
- 6. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49–e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vidan M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476–1482. [DOI] [PubMed] [Google Scholar]
- 8. Adunsky A, Lerner-Geva L, Blumstein T, Boyko V, Mizrahi E, Arad M. Improved survival of hip fracture patients treated within a comprehensive geriatric hip fracture unit, compared with standard of care treatment. J Am Med Dir Assoc. 2011;12(6):439–444. [DOI] [PubMed] [Google Scholar]
- 9. Nordstrom P, Michaelsson K, Hommel A, Norrman PO, Thorngren KG, Nordstrom A. Geriatric rehabilitation and discharge location after hip fracture in relation to the risks of death and readmission. J Am Med Dir Assoc. 2016;17(1):91.e1-e7. [DOI] [PubMed] [Google Scholar]
- 10. Neuman MD, Archan S, Karlawish JH, Schwartz JS, Fleisher LA. The relationship between short-term mortality and quality of care for hip fracture: a meta-analysis of clinical pathways for hip fracture. J Am Geriatr Soc. 2009;57(11):2046–2054. [DOI] [PubMed] [Google Scholar]
- 11. Deschodt M, Braes T, Broos P, et al. Effect of an inpatient geriatric consultation team on functional outcome, mortality, institutionalization, and readmission rate in older adults with hip fracture: a controlled trial. J Am Geriatr Soc. 2011;59(7):1299–1308. [DOI] [PubMed] [Google Scholar]
- 12. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349–1356. [DOI] [PubMed] [Google Scholar]
- 13. Lau TW, Fang C, Leung F. The effectiveness of a geriatric hip fracture clinical pathway in reducing hospital and rehabilitation length of stay and improving short-term mortality rates. Geriatr Orthop Surg Rehabil. 2013;4(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen P, Hung WW. Geriatric orthopedic co-management of older adults with hip fracture: an emerging standard. Ann Transl Med. 2015;3(16):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Penrod JD, Litke A, Hawkes WG, et al. Heterogeneity in hip fracture patients: age, functional status, and comorbidity. J Am Geriatr Soc. 2007;55(3):407–413. [DOI] [PubMed] [Google Scholar]
- 16. Ranhoff AH, Holvik K, Martinsen MI, Domaas K, Solheim LF. Older hip fracture patients: three groups with different needs. BMC Geriatr. 2010;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vochteloo AJ, Borger van der Burg BL, Tuinebreijer WE, et al. Do clinical characteristics and outcome in nonagenarians with a hip fracture differ from younger patients? Geriatr Gerontol Int. 2013;13(1):190–197. [DOI] [PubMed] [Google Scholar]
- 18. Kannegaard PN, van der Mark S, Eiken P, Abrahamsen B. Excess mortality in men compared with women following a hip fracture. national analysis of comedications, comorbidity and survival. Age Ageing. 2010;39(2):203–209. [DOI] [PubMed] [Google Scholar]
- 19. Endo Y, Aharonoff GB, Zuckerman JD, Egol KA, Koval KJ. Gender differences in patients with hip fracture: a greater risk of morbidity and mortality in men. J Orthop Trauma. 2005;19(1):29–35. [DOI] [PubMed] [Google Scholar]
- 20. Dripps R, Lamont A, Eckenhoff J. The role of anesthesia in surgical mortality. JAMA. 1961;178:261–266. [DOI] [PubMed] [Google Scholar]
- 21. Gosch M, Wortz M, Nicholas JA, Doshi HK, Kammerlander C, Lechleitner M. Inappropriate prescribing as a predictor for long-term mortality after hip fracture. Gerontology. 2014;60(2):114–122. [DOI] [PubMed] [Google Scholar]
- 22. Lehnert T, Heider D, Leicht H, et al. Review: health care utilization and costs of elderly persons with multiple chronic conditions. Med Care Res Rev. 2011;68(4):387–420. [DOI] [PubMed] [Google Scholar]
- 23. Cheng JS, Huang WF, Lin KM, Shih YT. Characteristics associated with benzodiazepine usage in elderly outpatients in Taiwan. Int J Geriatr Psychiatry. 2008;23(6):618–624. [DOI] [PubMed] [Google Scholar]
- 24. Jones SA, Bhandari S. The prevalence of potentially inappropriate medication prescribing in elderly patients with chronic kidney disease. Postgrad Med J. 2013;89(1051):247–250. [DOI] [PubMed] [Google Scholar]
- 25. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335–338. [DOI] [PubMed] [Google Scholar]
- 26. Stenvall M, Berggren M, Lundstrom M, Gustafson Y, Olofsson B. A multidisciplinary intervention program improved the outcome after hip fracture for people with dementia--subgroup analyses of a randomized controlled trial. Arch Gerontol Geriatr. 2012;54(3):e284–e289. [DOI] [PubMed] [Google Scholar]
- 27. McFarlane RA, Isbel ST, Jamieson MI. Factors determining eligibility and access to subacute rehabilitation for elderly people with dementia and hip fracture. Dementia (London). 2017;16(4):413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Juliebo V, Bjoro K, Krogseth M, Skovlund E, Ranhoff AH, Wyller TB. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57(8):1354–1361. [DOI] [PubMed] [Google Scholar]
- 29. Finsterwald M, Sidelnikov E, Orav EJ, et al. Gender-specific hip fracture risk in community-dwelling and institutionalized seniors age 65 years and older. Osteoporos Int. 2014;25(1):167–176. [DOI] [PubMed] [Google Scholar]
- 30. Neuman MD, Silber JH, Magaziner JS, Passarella MA, Mehta S, Werner RM. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174(8):1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635–M640. [DOI] [PubMed] [Google Scholar]


