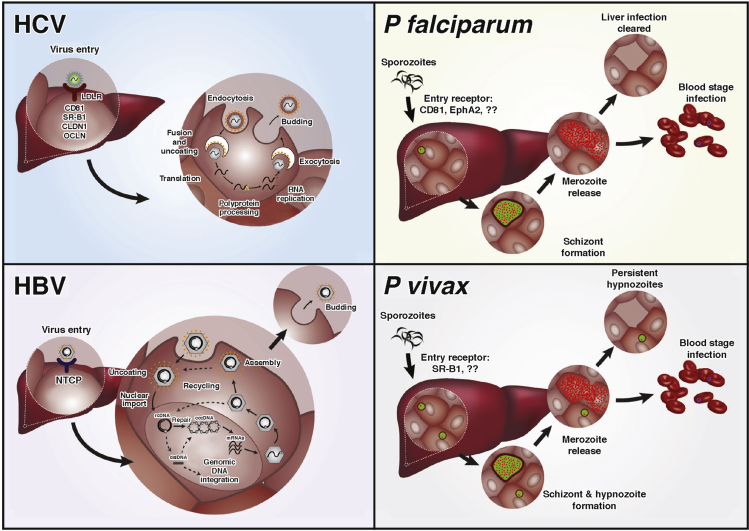
Figure 1.
Life cycles of 4 major human hepatotropic pathogens. HCV is a single-stranded, positive-sense RNA virus that belongs to the Flaviviridae family. Initial viral attachment to the hepatocyte membrane is mediated through glycosaminoglycans and the LDL receptor. Interactions with other host factors CD81, scavenger receptor class B member 1 (SRB1), claudin 1 (CLDN1), occludin (OCLDN), and possibly other molecules, such as CLDN9, CLDN6, EphA2, and epidermal growth factor receptor, are required for cell entry. Clathrin-mediated endocytosis of the virus is followed by fusion of the viral and endosomal membranes, resulting in the release of nucleocapsid into the cytoplasm. Positive-strand genomic RNA is released into the cytosol on uncoating of the viral nucleocapsid, which initiates synthesis of the HCV polyprotein. Host cell lipid synthesis pathways are tightly linked to the later stages of assembly and virus release. HBV is a DNA virus that belongs to the family Hepadnaviridae. HBV enters the hepatocyte via the sodium/bile acid cotransporter NTCP.23 After uncoating, the partially relaxed double-stranded circular viral DNA (rcDNA) is directed to the nucleus where viral DNA lesions are repaired by the host machinery, converting into covalently closed circular DNA (cccDNA), which serves as a template for viral RNA production. Five transcripts are made that encode envelope, core and X antigens, viral polymerase, and pregenomic RNA (pgRNA). pgRNA can be reverse transcribed into rcDNA, which is assembled with the viral capsids and released from the host cell. During reverse transcription of pgRNA double-stranded linear (dsl) DNA can be formed and are capable of integration into human chromosomes. Plasmodium falciparum and Plasmodium vivax are apicomplexan parasites. Plasmodium sporozoites are deposited into the human skin via bite of an infected Anopheles mosquito and travel to the liver where they invade hepatocytes. CD8138, 148 and EphA2106 for P falciparum, and more recently SR-B1134 for P vivax, have been implicated as required entry factors. On invasion of hepatocytes, parasites differentiate and divide by schizogony to form thousands of progeny, merozoites, which are released into the bloodstream where they can cyclically invade red blood cells, initiating the blood stage of the disease. P vivax has an additional, unique aspect of its liver development where a subset of the parasites, called hypnozoites, remain dormant and can reactivate weeks to years after the initial infection to reinitiate disease.