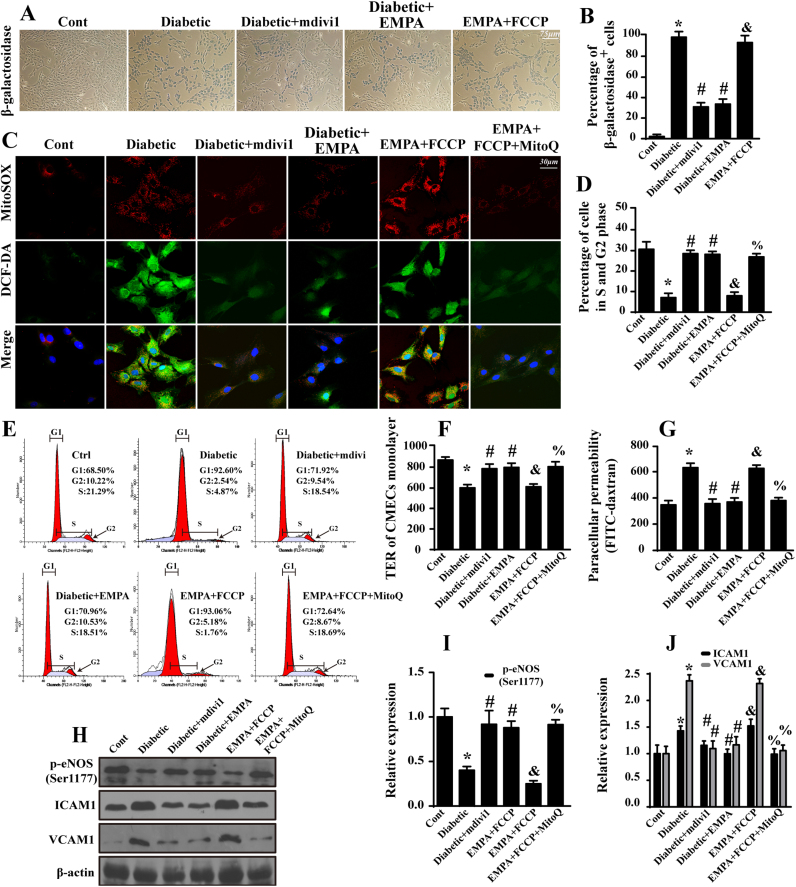
Fig. 5.
Empagliflozin protects CMECs against cellular senescence and barrier dysfunction by suppressing fission-mediated mtROS overproduction. A. Representative images of β-galactosidase staining, which was the marker for aged cells. B. Quantification of β-galactosidase-positive endothelial cells. C. The immunofluorescence of intracellular ROS and mtROS as assessed by DCF-DA and MitoSOX™ Red Mitochondrial Superoxide Indicator. Mitoquinone (MitoQ, 2 μM) was applied for 24 h to reduce the cellular oxidative damage. D–E. Cell cycle distribution was detected by performing flow cytometric quantification. Loss of fission due to empagliflozin and mdivi1 contributed to the cell cycle transition from G0/G1 to S phase, suggesting the anti-senescence effects of empagliflozin occur via fission inhibition. F. A TER assay was performed to detect CMEC barrier function during mitochondrial fission. TER increases when endothelial cells adhere and spread out and decreases when endothelial cells retract or lose adhesion, reflecting endothelial barrier integrity. G. FITC-dextran clearance was measured to assess changes in endothelial permeability. FITC-dextran was applied on top of the inserts and allowed to permeate through cell monolayers. The increased endothelial permeability resulted in the retention of more FITC-dextran. Thus, FITC content remaining in the plate indicates the extent of CMEC permeability. H–J. The change of expression of p-eNOS (Ser1177), ICAM1 and VCAM1. Mean ± SD, *P < 0.05 vs. Control (Cont) group; #P < 0.05 vs. Diabetic group, &P < 0.05 vs. Diabetic + EMPA group, %P < 0.05 vs. Diabetic + EMPA + FCCP group.