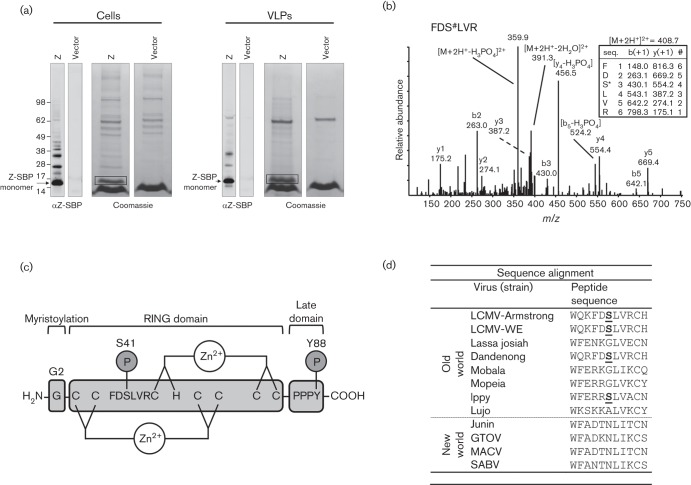
Fig. 1.
The LCMV matrix protein Z is phosphorylated at S41. (a) HEK-293T cells were transfected with a plasmid encoding SBP-tagged LCMV strain Armstrong Z or an empty vector. Two days later cells and virus-like particle (VLP)-containing supernatant were lysed and Z-SBP was affinity purified using streptavidin-coated magnetic beads. The purified Z-SBP was subjected to SDS-PAGE and detected by Western blotting using an anti-SBP tag antibody or Coomassie stain. The presumptive monomeric Z bands from cells or VLPs were excised from the Coomassie stained gels (as indicated by the boxes) and subjected to reduction, alkylation and in-gel tryptic digestion prior to mass spectrometry analysis of extracted peptides. (b) A representative low energy collision-induced dissociation tandem mass spectrum with its corresponding fragment ion table from low energy collision-induced dissociation of a Z-derived tryptic peptide (FDS#LVR) containing the phosphorylated S41 where # denotes phosphorylation. The fragment ion table lists the predicted m/z values of the singly charged b and y ions. Major measured b and y ions, as well as dominant losses of phosphoric acid are labelled. Phosphoric acid loss is a major signature in tandem mass spectra of phosphoserine/threonine-containing peptides. (c) Cartoon of the LCMV Z protein depicting the G2 myristoylation site, the central zinc-binding RING domain and the C-terminal PPPY late domain. The S41 phosphorylation site and its flanking amino acids as well as the previously described Y88 phosphorylation site (Ziegler et al., 2016) are indicated. (d) Alignment of Old and New World arenavirus Z protein sequences shows that S41 (bold and underlined for LCMV strains Armstrong and WE) is conserved with the Old World arenaviruses Dandenong and Ippy virus.