Abstract
Epidemiology studies have found that a comorbidity exists between traumatic brain injury (TBI) and stress-related disorders. However, the anatomical and cellular bases for this association is poorly understood. An inability to extinguish the memory of a traumatic event lies at the core of many stress-related disorders. Experimental studies have shown that the medial pre-frontal cortex (mPFC), especially the infralimbic (IL) cortex, is required for extinction and for storing the memory of extinction. The output from the central nucleus of amygdala projects to the lateral hypothalamus, paraventricular nucleus, and central gray to regulate heart rate, stress hormone release, and freezing behavior, respectively. Projection neurons of the IL (layers II/III pyramidal neurons) are thought to stimulate GABAergic neurons in the amygdala, which, in turn, inhibit central amygdala output and reduce fear expression. Thus, loss and/or altered morphology of projection neurons of IL as a result of a mild TBI (mTBI) can compromise their ability to effectively inhibit the central amygdala, allowing the original fear memory to drive behavior. Using lateral mild fluid percussion injury (mFPI) in rats, we found that mFPI did not reduce neuronal numbers in the IL, but caused a significant reduction in overall dendritic spine density of both basal and apical dendrites on layer II/III pyramidal neurons. Spine numbers on layer V/VI pyramidal neurons were not significantly changed as a result of mFPI. The reduction in spine density on layer II/III pyramidal neurons we observed may diminish the efficacy of these neurons to inhibit the output of the central amygdala, thereby reducing the ability of the IL to suppress fear responses after extinction training. Consistent with this, mFPI rats display enhanced freezing behavior during and after extinction training as compared to sham-operated controls, although the ability to form contextual fear memories was not impaired. These results may have implications in stress-related disorders associated with mTBI.
Keywords: : concussion, fear extinction, medial prefrontal cortex, post-traumatic stress disorder, PTSD
Introduction
Traumatic brain injury (TBI) increases the risk for developing psychiatric illnesses.1 Although depression is the most prevalent psychiatric disorder observed in people who have sustained a TBI, anxiety disorders are also common and are frequently comorbid with depression.2 Studies have shown that individuals with TBI experience a variety of anxiety disorders, including general anxiety disorder, panic disorder, obsessive compulsive disorder, and post-traumatic stress disorder (PTSD).1,3 Consistent with this, epidemiological studies have reported comorbidity between mild TBI (mTBI) and PTSD. Among military personnel and veterans with TBI, estimates of PTSD range from 12% to 89%.4 Even when accounting for pre-deployment symptoms, prior TBI, and combat intensity, TBI during the most recent deployment is the strongest predictor of post-deployment PTSD symptoms.5 These results suggest that TBI may make a person vulnerable to (or reduce the threshold for) developing PTSD or other stress disorders in response to a subsequent traumatic/life-threatening event.
An inability to extinguish the memory of a traumatic event lies at the core of many stress-related disorders.6 When a person experiences a traumatic event, the subject can form an association between an otherwise innocuous stimuli (referred as the conditioned stimulus) such as a sound, a smell, and/or the context in which the event occurred, and the harmful event (referred to as the unconditioned stimulus). This association is learned rapidly, and the memory for the traumatic event is robust and can be long-lasting. When the person is subsequently exposed to the conditioned stimulus alone (referred to as the trigger), it causes a fear response referred to as the conditioned response. Repeated exposure to the conditioned stimulus in the absence of the unconditioned stimulus results in a gradual reduction of the fear response through extinction. In the late 1920s, Pavlov suggested that extinction does not erase the stored memory, but results from new learning.7 Consistent with this, it has been demonstrated that the fear response returns when the conditioned stimulus is presented in a new context.8–10 This indicates an important role for the context in which the extinction training is carried out in fear memory extinction. These and other studies indicate that extinction forms a new memory that suppresses learned fear responses.
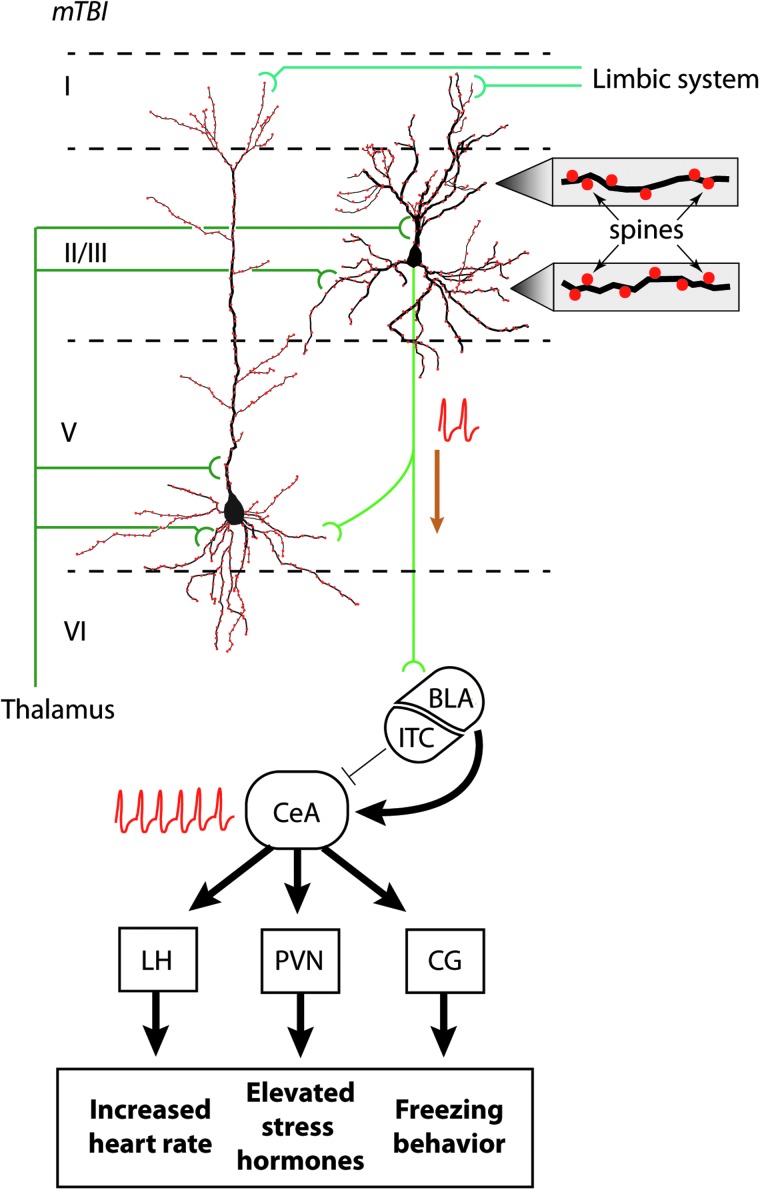
The amygdala is a key structure that is essential for the formation of fear memories and for mediating fear responses. The neurons of the central nucleus of amygdala (CeA) project to structures including the periaqueductal gray (controls the freezing behavior), the lateral hypothalamus (increases blood pressure), and the paraventricular nucleus of the hypothalamus (mediates release of stress hormones). The output from the central nucleus, in turn, is controlled by the projections from pyramidal neurons within the infralimbic (IL) cortex of the medial pre-frontal cortex (Fig. 1). When the activity of IL neurons is blocked, animals can learn the association between the conditioned and unconditional stimuli; however, the memory of extinction is impaired.11,12 In contrast, stimulation of IL neurons facilitates extinction learning and the formation of extinction memory.12–14 Based on in vivo activity recording and anatomical tracing data, a model by which IL neurons are involved in fear memory extinction has been proposed. In this model, repeated exposure to the conditioned stimulus activates pyramidal neurons within the IL. IL neurons directly (or indirectly through the basolateral amygdala) activate the intercalated cells of the amygdala, which are GABAergic neurons located between the basolateral and the central amygdalas.15,16 These cells then inhibit central amygdala activity, thereby suppressing the fear response.
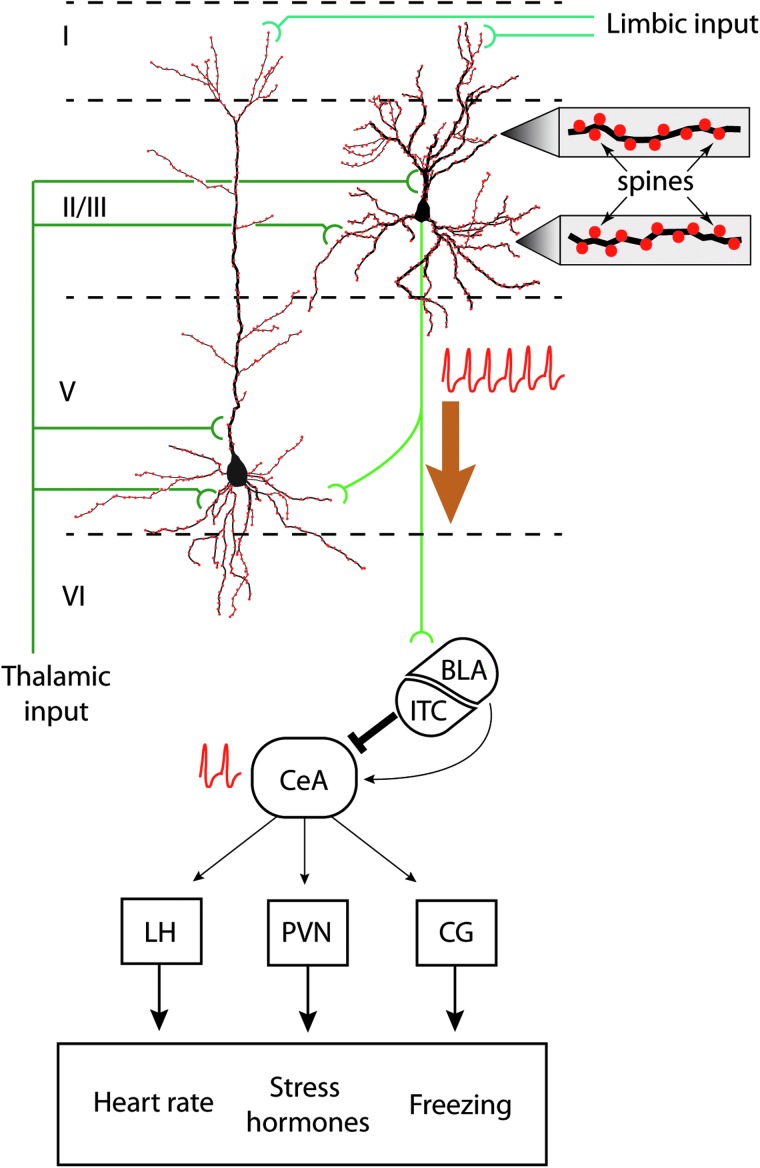
FIG. 1.
IL regulation of fear memory extinction. Schematic drawing of layer II/III and layer V pyramidal neurons within the IL. Based on cortical neuroanatomy, layer II/III and layer V neurons receive limbic inputs (from the amygdala, hippocampus, entorhinal cortex, and cingulate cortex) to the distal portions of their apical dendrites, whereas thalamic input is received on proximal apical and basal dendrites. Spines (filled red circles) cover the dendrites, an enlarged view of which is shown to the right. During extinction, layer II/III neurons fire (indicated by train of action potentials) upon exposure to the CS alone. The excitatory output of these neurons directly and/or indirectly activate GABAergic neurons that comprise the intercalated cells of the amygdala (ITC). The ITC, in turn, inhibits the central nucleus of the amygdala (CeA). Reduced CeA activity (indicated by action potentials) results in less stimulation of the lateral hypothalamus (LH), the paraventricular nucleus (PVN), and the central gray (CG), thereby reducing the physical manifestations of fear. BLA, basolateral amygdala; IL, infralimbic cortex.
Although epidemiological studies have reported comorbidity between TBI and stress-related disorders, the cellular mechanism(s) that underlie this association is not known. Given that spine density is a correlate for the strength of neuronal communication, we questioned whether mFPI alters the density of spines on apical or basal dendrites in layer II/II or layer V/VI pyramidal neurons of the IL. Our results show that lateral mild fluid percussion injury (mFPI) impaired extinction learning and extinction memory when tested 1 month after the injury, in the absence of cell loss in the IL. In addition, we observed a significant reduction in the spine density of layer II/III pyramidal neurons.
Methods
Materials
Male Sprague-Dawley rats (275–300 g) were purchased from Harlan Laboratories (Indianapolis, IN). Antibodies to neuronal nucleus (NeuN; Millipore, Billerica, MA) and CaMKIIα (Cell Signaling Technology, Danvers, MA) were obtained for use in these studies. An FD Rapid GolgiStain Kit was purchased from FD Neurotechnologies (Columbia, MD).
Lateral fluid percussion injury
All experimental procedures were approved by the Institutional Animal Care and Use Committee and were conducted in accord with the recommendations provided in the Guide for the Care and Use of Laboratory Animals. Protocols were designed to minimize pain and discomfort during the injury procedure and recovery. Lateral FPI was carried out similar to that described previously.17–19 Briefly, rats were initially anesthetized using 5% isoflurane with a 1:1 N2O/O2 mixture and then maintained with a 2.5% isoflurane with 1:1 air/O2 mixture by a face mask. Animals were mounted on the stereotaxic frame; a 4.8-mm-diameter craniotomy was carefully made midway between bregma and lambda. The craniotomy used for hub placement was centered at 4.4 mm from midline, placing the epicenter of the injury over the right parietal cortex. A hub (modified from a 20-gauge needle) was implanted into the burr hole and affixed to the skull by contact adhesive and dental cement. Once the assembly was secured, the rat was removed from the anesthesia and allowed to regain its toe pinch reflex. Immediately upon regaining this reflex, the rat was injured using an FPI device and a pressure of 1.5 atmosphere (atm) over base room pressure. Previous studies have shown that this injury magnitude does not cause visible brain damage, nor overt hippocampal damage, but does cause axonal injury as detected by diffusion tensor imaging.20 Acute neurological responses were recorded post-injury. On average, injured animals regain their response to toe pinch at 72.4 ± 5.6 sec, tail pain reflexes by 100.1 ± 5.3 sec, and their righting response (ability to right itself three consecutive times after being placed on its back) by 387 ± 11.2 sec. By comparison, sham-operated animals require only 36.3 ± 8.4 sec to regain their righting response (after subtraction of the tail pinch reflex). Post-injury, the hub and surrounding dental cement were immediately removed and the incision closed by wound clips. Sham-operated animals received all the aforementioned surgical procedures except hub implantation and the injury. Animals' body temperature was maintained at 37°C during the surgery using a rectal thermometer coupled to a heating pad.
Immunohistochemistry
Two weeks after injury or sham operation, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg) and transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Brains were removed, post-fixed overnight in perfusant, then cryoprotected in a 30% sucrose solution. Tissue sections (40 μm in thickness) were generated using a Leica CM 1950 cryostat (Leica Biosystems Nussloch GmbH, Nussloch, Germany), and stored in PBS at 4°C until needed. Free-floating slices were incubated overnight in primary antibody (0.5–1.0 μg/mL) in 0.1% Tween-20 in PBS containing 2% bovine serum album and 2.5% normal goat serum at 4°C. After extensive washing, immunoreactivity was detected using species-specific secondary antibodies conjugated to horseradish peroxidase and developed using diaminobenzadine as the chromagen.
Stereological cell counts
A blind counting methodology was used for determination of neuronal density in the IL. Sections spanning the rostral-caudal extent of the IL were chosen and immunostained as described above. Immuno-positive cells were counted using the optical dissector technique and Stereo Investigator (MicroBrightField Bioscience, Williston, VT).21 The IL was identified based on its relative position to major landmarks, such as the forceps minor and genu of the corpus callosum, and the nucleus accumbens as indicated in Paxinos and Watson.22 The IL was further differentiated from the pre-limbic cortex because of its thinner shape and fewer, less well-defined cortical layers.23 The periphery of the IL was carefully outlined and the number of labeled cells in approximately 20 computer-chosen areas scored for each section by a blinded observer. The counting frame was 60 × 60 μm. The size of the counting frame and the number of grid sections were determined based on preliminary cell counts. Cells in the outermost planes of focus were omitted to avoid counting cell caps. The number of labeled cells/mm2 for each section was obtained from the estimated cells divided by the contour area. The number of cells/mm2 for each animal was calculated as the average of the number of cells/mm2 from each section examined.
Context fear conditioning and extinction
Beginning on day 28 post-injury, rats were trained in a one-trial fear conditioning task. Rats were placed in the training chamber and allowed to freely explore their new surroundings for a period of 2 min. Freezing behavior was monitored (in 2-sec increments) throughout the 2-min period by an observer blinded to the injury status of the animals. A 2-sec, 0.7-mA footshock was then delivered. Thirty seconds after the footshock, rats were removed from the training chamber and returned to their home cage. Twenty-four hours later, fear memory was tested by placing the animal back in the training chamber for a period of 3 min and scoring freezing behavior. Extinction training was carried out 24 h after fear memory testing by placing the animal in the training chamber for a period of 10 min. Freezing behavior was monitored throughout the extinction period. Twenty-four hours later, memory for the extinction was tested as described above.
Rapid Golgi staining
Rapid Golgi staining was performed using a Rapid GolgiStain kit (FD Neuro Technologies, Ellicot City, MD) following the procedures recommended by the manufacturer. Thirty-two days post-injury (after the completion of extinction memory testing), animals were killed by decapitation, brains were quickly extracted, and the frontal lobe removed while submerged in ice-cold artificial cerebrospinal fluid. The frontal lobe was rinsed in ice-cold water, then cut into 5-mm slabs. Slabs were immersed in silver impregnation solution for 2 weeks in the dark, followed by immersion in “Solution C” for an additional week. Next, 150-μm-thick serial sections spanning the rostral-caudal extent of the IL were cut on a cryostat. Sections were mounted on 3% gelatin-coated slides and allowed to dry overnight. Development was carried out as described by the vendor, followed by fixation in 1% glutaraldehyde for 1 h. Sections were rinsed then dehydrated using an alcohol series and clarified using xylene before cover-slipping with Permount (Thermo Fisher Scientific Inc., Waltham, MA). Silver-impregnated layer II/III and V/VI pyramidal neurons in the IL were identified by their characteristic triangular cell soma with a prominent apical dendrite projecting toward the superficial layers of the cortex. Layer II/III was identified as the neuron dense subcortical layer proximal to the neuron sparse layer I, whereas layer V/VI was identified by the presence of pyramidal neurons with relatively larger cell bodies. Five pyramidal neurons in the IL ipsilateral to the injury per animal were chosen randomly (n = 5 for both injured and sham animals). The number of spines on first- to fourth-order apical and basal dendrites were counted by observers blind to the group designations. To be included in the analysis, the dendritic branch had to be at least 25 μm in length. The number of spines was divided by the length of the dendrite to give spine density.
Statistical analysis
Cell counts between sham and mFPI animals were statistically compared using a Student's t-test for unpaired groups. Evaluation of spine density was compared using a two-way analysis of variance (ANOVA). Group main and interactions (group × branch order) were considered to be statistically different at p < 0.05. For evaluation of behavioral data, repeated measures (RM) ANOVAs (two-way or one-way as appropriate) and t-tests were utilized to determine statistical differences. A Holm-Sidak method for multiple comparisons post-hoc test was used to determine data points with significant differences. For data that did not pass a Shapiro-Wilk normality test, appropriate nonparametric analysis was performed. Data were considered significant at p ≤ 0.05 and presented as mean ± standard error of the mean.
Results
Mild fluid percussion injury impairs fear memory extinction
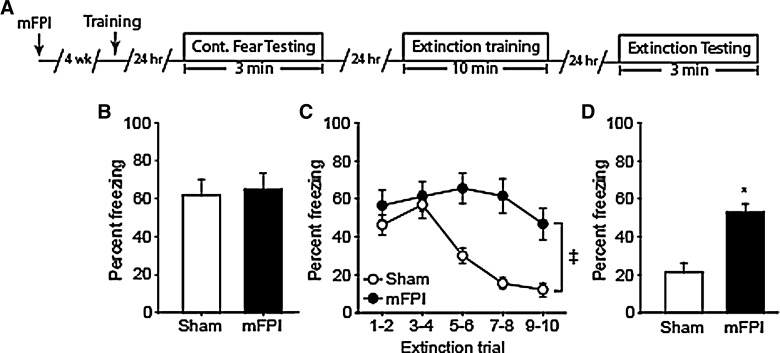
Rats were subjected to either a 1.5-atm mFPI or sham-operated (n = 8/group). Twenty-eight days after the injury, animals were trained using a one-trial context fear paradigm (Fig. 2A) in the absence of a salient cue (e.g., tone). Twenty-four hours later, fear memory was assessed by placing the animals into the training chamber and measuring percent freezing over a 3-min period in the absence of a footshock. Freezing behavior, defined as the absence of all movement except that needed for respiration, was scored in 2-sec intervals and used as an indicator of fear. Figure 2B shows that there was no significant difference in freezing behavior between the mFPI and sham animals (p = 0.797), indicating that both groups learned to associate the footshock with the training chamber.
FIG. 2.
Lateral mFPI impairs extinction and memory for extinction. (A) Timeline for contextual fear and extinction training and testing. Rats received a lateral mild FPI (or sham operated); 4 weeks later, animals were trained in a one-trial context fear conditioning task. Twenty-four hours after training, memory was assessed. Fear memory extinction was carried out 24 h later. Memory of the extinction was tested 24 h later. Freezing behavior (percent time spent freezing) was used as an indicator of fear. (B) Both sham and mFPI animals showed similar freezing behaviors during the period of context fear testing, an indication that mFPI does not impair context fear memory. (C) During extinction training, sham-operated rats learn that the context does not predict the footshock, as indicated by reduced freezing over time. mFPI animals displayed enhanced freezing behavior throughout the extinction training period, indicating impaired extinction learning. (D) When tested for extinction memory, sham animals displayed reduced freezing indicating memory for the extinction learning. In contrast, mFPI animals displayed significantly enhanced freezing, indicating impaired memory for the extinction training. Data are mean ± standard error of the mean. *Significant difference between sham and mFPI by t-test. ‡Significant difference between sham and mFPI by repeated-measures two-way analysis of variance. mFPI, mild fluid percussion injury.
Extinction training was carried out by placing the animals in the training chamber for a period of 10 min in the absence of the footshock. Freezing behavior was scored in 2-sec intervals, then binned into 2-min intervals for analysis. Figure 2C shows that over the 10-min extinction period, sham-operated animals learn that the training chamber is now “safe” as indicated by a significant reduction in their freezing behavior over time (one-way RM ANOVA, F(4,28) = 20.106; p < 0.001). mFPI animals, by comparison, displayed a modest reduction in freezing behavior (one-way RM ANOVA, F(4,28) = 2.676; p = 0.052), indicating impaired extinction learning. When the acquisition curves for extinction learning were compared across groups, a significant difference was observed between the sham and mFPI groups (interaction by two-way RM ANOVA, F(4,56) = 8.463; p < 0.001). Memory for extinction was tested by placing the animal back in the training chamber 24 h after extinction training. Sham-operated rats displayed freezing behaviors during only 20% of the testing period (Fig. 2D). In contrast, mFPI rats displayed freezing behaviors 50% of the time, indicating a poor memory for the extinction training. When freezing behaviors were compared, mFPI animals froze significantly more than sham-operated controls (p < 0.001). Taken together, these results indicate that mild lateral FPI does not impair the ability of injured animals to form context fear association memories, but impairs context fear memory extinction and memory of extinction.
Mild fluid percussion injury does not cause neuronal loss in the infralimbic cortex
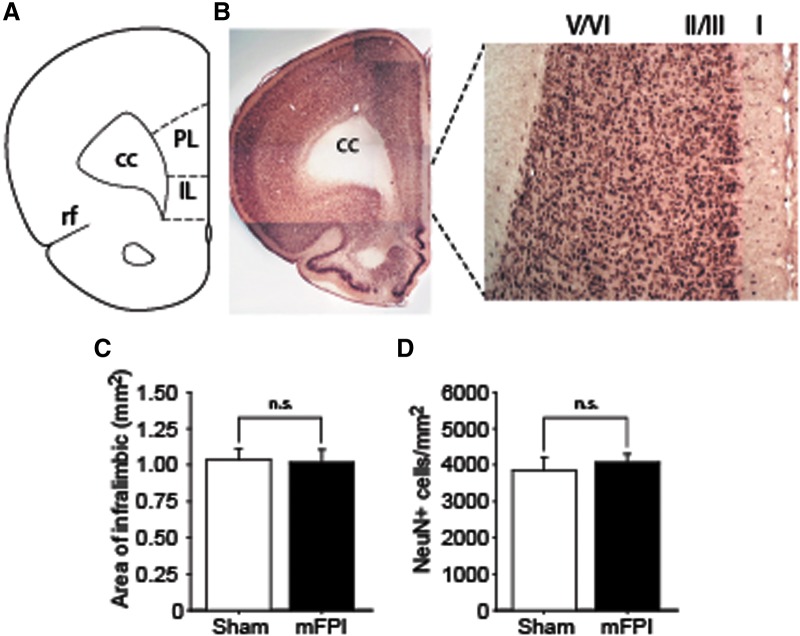
Previous studies have demonstrated a key role for the IL in the formation of fear extinction memory.11,12,14 We therefore examined whether lateral mFPI causes the loss of neurons within the IL. Figure 3A shows the relative position of the IL as described by Paxinos and Watson.22 Tissue sections from sham and 14-day post-injury mFPI animals containing the IL (Fig. 3B) were used for NeuN (a marker of neurons) immunohistochemistry and immunopositive cells counted using the optical dissector technique. Figure 3C shows no significant differences in the area of the ipsilateral IL (p = 0.824). When NeuN-positive cells in the IL were counted, there was no significant difference (p = 0.617) between the sham and mFPI groups (n = 4/group), indicating that mFPI does not cause the loss of IL neurons.
FIG. 3.
Lateral mFPI does not cause neuronal cell loss in the infralimbic (IL) cortex. (A) Drawing indicating the relative position of IL cortex.22 cc, corpus callosum; PL, pre-limbic cortex; rf, rhinal fissure. (B) Representative images of NeuN-stained sections showing the neuronal layers of the IL. (C) Summary data showing that the area of the IL measured in sham and mFPI rats (n = 4/group) did not differ between groups. (D) Stereological cell counts of the ipsilateral IL showed that the number of NeuN-positive neurons/mm2 did not significantly change as a result of mFPI (n = 4/group). mFPI, mild fluid percussion injury; NeuN, neuronal nucleus.
Mild fluid percussion injury decreases spine density in layer II/III pyramidal neurons of the infralimbic cortex
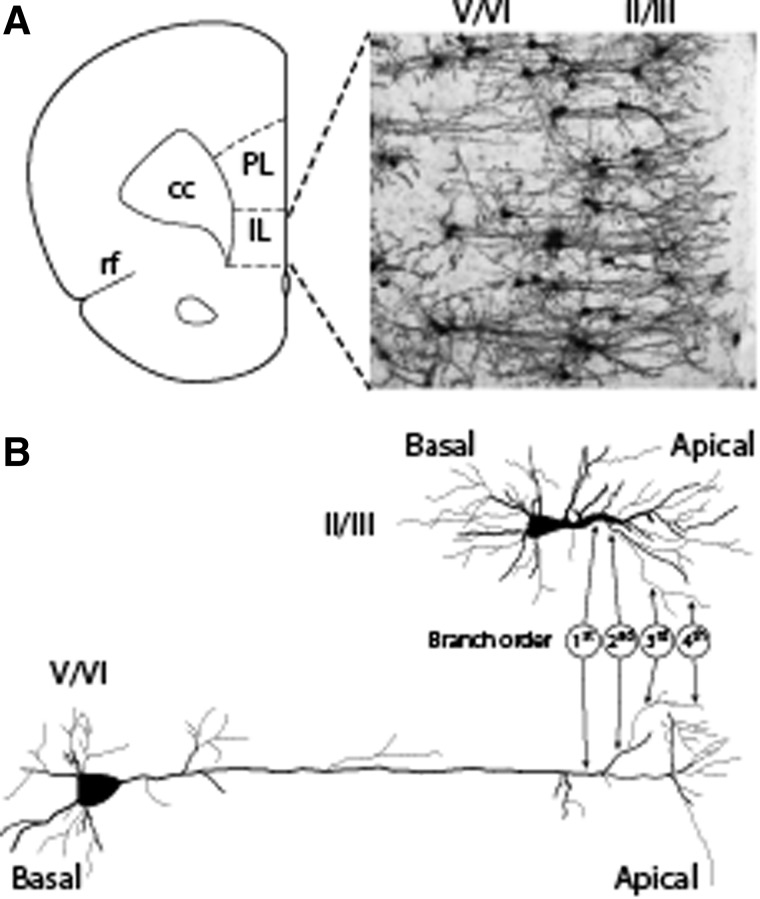
Retrograde tracing studies have demonstrated that IL pyramidal neurons projecting to the amygdaloid complex are primarily located in layers II/III and V/VI.24,25 In order to assess whether morphological changes occurred as a result of mFPI, injured and sham-operated animals were euthanized 32 days post-injury (corresponding to the end of extinction training and testing) and tissue containing the IL processed using a Rapid Golgi staining kit (Fig. 4A). The number of spines on layer II/III and layer V/VI apical and basal dendrites were counted by observers blind to the groups. Figure 4B shows a representative drawing of a typical layer II/III pyramidal neuron. These cells were identified based on their proximity to the neuron sparse layer I, their characteristic triangular soma, and their relatively short apical dendrites. Because layer II and layer III are difficult to distinguish using Golgi staining, the pyramidal neurons in these layers were combined for analysis. Similarly, pyramidal neurons within layers V and VI, identified by their relatively larger triangular soma and long apical dendrites, were combined for analysis. Spines on first- to fourth-order apical and basal dendrites were counted (indicated in Fig. 4B), the length of the dendrite measured, and the density calculated as described in the Methods section.
FIG. 4.
Identification of Golgi stained layer II/III and layer V/VI neurons within the infralimbic cortex (IL). (A) Illustration showing the relative position of the IL compared to the pre-limbic cortex (PL), the corpus callosum (cc), and the rhinal fissure (rf). A representative image of a Golgi-stained IL showing the position of layer II/III and layer V/VI neurons is shown. (B) Tracings of Golgi-stained layer II/III and layer V/VI pyramidal neurons from the IL showing the characteristic morphology (triangular shaped soma) to the cells in each layer. First- (dendrites emerging directly from the cell soma), second- (dendrites emerging from the first order), third- (dendrites emerging from the second-order dendrites), and fourth-order (dendrites emerging from the third order) dendrites for both layer II/III and layer V/VI apical dendrites are indicated. Spines on both basal and apical (labeled) dendrites were counted.
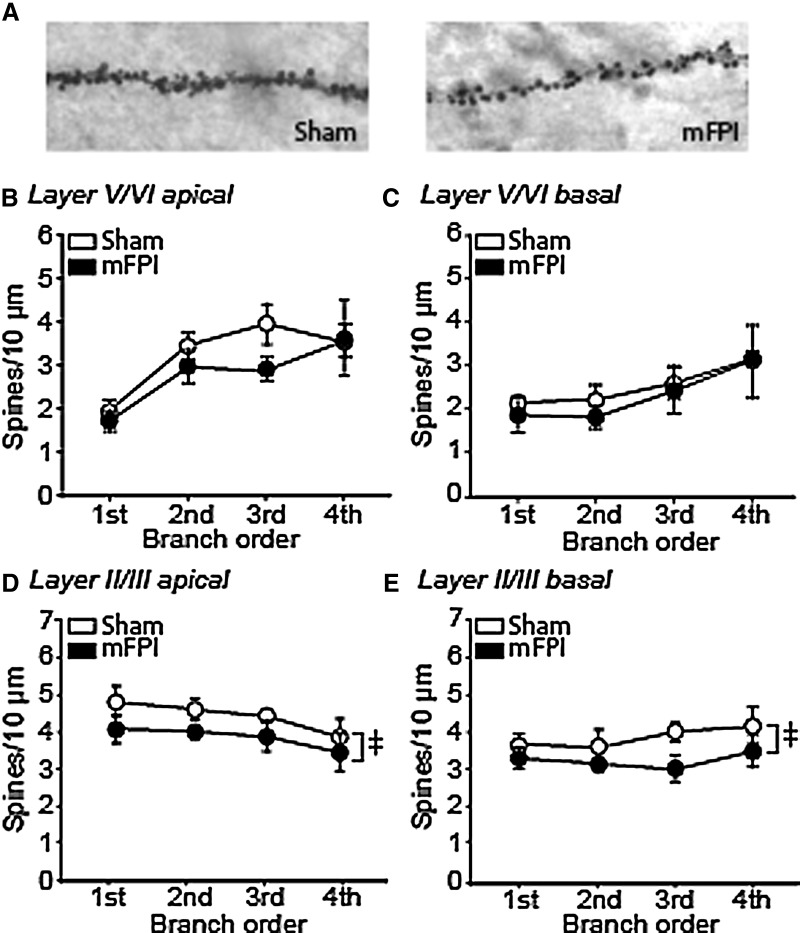
Representative photomicrographs of dendritic spines (third-order apical dendrites from layer II/III pyramidal neurons) from a sham and an mFPI animal are shown in Figure 5A. When analyzed using a two-way ANOVA, no significant differences in spine density were detected in either the apical (Fig. 5B; F(1,3) = 2.374; p = 0.132) or basal (Fig. 5C; F(1,3) = 0.491; p = 0.488) dendrites of layer V/VI pyramidal neurons. In contrast, a significant reduction in overall dendritic spine density was observed on both the apical (Fig. 5D; F(1,3) = 6.001; p = 0.020) and basal (Fig. 5E; F(1,3) = 7.325; p = 0.011) dendrites of layer II/III neurons. Post-hoc analysis indicated that the decrease in spine density was not restricted to any particular branch order.
FIG. 5.
Lateral mFPI significantly reduces overall spine density in layer II/III pyramidal neurons of the IL. (A) Representative images of third-order apical dendrites and spines from a sham and an mFPI animal. Summary data showing of the number of spines/10 μm counted in first- to fourth-order (B) apical and (C) basal dendrites from layer V/VI pyramidal neurons from sham and mFPI rats. (D) Summary data showing that mFPI causes a significant overall, but not on individual branch orders, decrease in spine density (spines/10 μm) on apical dendrites from layer II/III pyramidal neurons compared to sham animals. (E) Significant decreases in overall (but not on individual branch orders) spine density (spines/10 μm) on basal dendrites from layer II/III pyramidal neurons were observed in mFPI animals compared to sham controls. Data are mean ± standard error of the mean. ‡Significant difference between sham and mFPI by two-way analysis of variance. mFPI, mild fluid percussion injury.
Discussion
Previous epidemiological studies have reported an association between stress-related disorders and mTBI. However, the cellular basis for this association is not known. Our study revealed two key findings: 1) mFPI caused impaired context fear extinction and memory of extinction, and 2) this impairment was not attributed to loss of neurons within the IL, but was associated with an overall reduction in apical and basal dendritic spine density of layer II/III pyramidal neurons. Taken together, these results suggest that mTBI may compromise IL cortex function and may make a person susceptible to stress-related disorders by impairing extinction learning and/or extinction memory. To our knowledge, this is the first study to show that mTBI causes impairment of contextual fear memory extinction and induces changes in the morphology of neurons in the IL.
Pharmacological, electrical, and optogenetic manipulations of IL function have demonstrated its involvement in extinction learning and extinction memory.11,26–29 Fear memory extinction has been proposed to occur through IL-mediated activation of GABAergic neurons within the intercalated cells, thereby suppressing the ability of the basolateral amygdala to activate the central amygdala (Fig. 1). An alternate model for fear memory extinction posits that a primary target of the IL is a distinct population of neurons in the basolateral amygdala, rather than the intercalated cells.30 In this model, IL modulates amygdala output through the basolateral amygdla (through the intercalated cells) to suppress fear.29,31,32 Although it has been previously reported that lateral mild FPI (centered −4.5 mm from bregma) does not cause overt cell loss in the hippocampus,20,33,34 it had not been examined whether this injury results in damage to the IL. Unbiased stereological cell counts of NeuN-stained sections revealed that mFPI does not cause any significant loss of neurons in the IL. However, quantification of dendritic spines revealed a significant reduction in overall spine density in both apical and basal dendrites of pyramidal neurons in layers II/III. One interesting aspect of the present study is that we found the spine density of layer II/III, but not layer V/VI, pyramidal neurons in the IL was decreased as a result of mFPI. Although the reason for this is not clear, given that layer V/VI pyramidal neurons receive inputs from layer II/III pyramidal neurons, it is anticipated that the changes we observed in layer II/III would alter the output of layer V/VI neurons as well.
Fear learning and memory have been previously tested in animals subjected to lateral mFPI. For example, Reger and colleagues tested the consequences of mFPI on fear acquisition and memory using different training paradigms.35 Although this group demonstrated that training paradigms that used an auditory cue resulted in increased fear memory, no difference in fear memory was observed between sham and mFPI animals when tested after contextual fear conditioning. This is consistent with our results using a one-trial contextual fear conditioning paradigm (no auditory cue was presented) in which both sham and mFPI animals displayed equivalent freezing behaviors when placed back into the training chamber 24 h after conditioning (Fig. 2B). Although mFPI animals were capable of making long-term contextual fear memories, they had poor extinction learning as indicated by modest decrease in freezing over time (Fig. 2C). In contrast, the freezing behavior of sham animals significantly decreased over time as a result of extinction learning. Comparison of freezing behaviors 24 h after extinction learning as an indicator of memory for extinction revealed that mFPI animals froze significantly more than sham-operated controls (Fig. 2D), indicating poor memory of extinction. Using a controlled cortical impact model of TBI, Sierra-Mercado and colleagues did not observe any influence of injury on fear memory extinction or extinction memory, a finding in contrast to that presented here. Although the reason for this apparent discrepancy is not known at present, differences in injury (focal vs. diffuse), cued versus noncued training, and time of testing could have contributed. Future studies will be required to delineate the relative contribution of these differences in the results we observed.
One limitation of the current study is that although we demonstrate an association between reduced spine numbers and impaired fear memory extinction, we cannot establish causality. Intracellular recordings in vivo from IL neurons with altered morphology would be required to determine the impact of the changes we observed on neuronal activity. A similar approach has been used to demonstrate the consequences of altered hippocampal neuronal morphology in post-traumatic epilepsy.36,37 In summary, our results show that lateral mFPI does not cause neuronal loss in the IL, but significantly reduces overall spine density of layer II/III pyramidal neurons. This decrease was associated with impaired extinction learning and extinction memory, suggesting that the reduced spine density may have compromised the efficacy of the IL to inhibit output from the central amygdala (Fig. 6). The reduced efficacy of the IL as a result of mTBI may make the injured brain vulnerable (or reduce the threshold) for developing stress-related disorders. These results further suggest that a history of mTBI, in which the function of the prefrontal cortex is compromised, may increase the likelihood for developing PTSD-like symptoms to a subsequent traumatic event.
FIG. 6.
Hypothetical model for impaired extinction and extinction fear memory after mild TBI (mTBI). Layer II/III pyramidal neurons in the IL receive information regarding the context via projections from limbic structures (e.g., amygdala, hippocampus, entorhinal cortex, and cingulate cortex) which synapse on their dendrites. In addition, these neurons also receive thalamic input predominately on their proximal dendrites. A reduction in spine density of these neurons as a result of mTBI is likely decrease the ability of these neurons to initiate action potentials. This reduction in firing rate will decrease the ability of the IL to directly or indirectly stimulate the inhibitory GABAergic neurons in the intercalated cells of the amygdala (ITC). As a result, the output from the CeA is expected to remain elevated, leading to physical manifestations of fear (e.g., heart rate, freezing behavior, and stress hormones). This model also predicts that reduced activity in layer II/III neurons will decrease the output from layer V/VI neurons. BLA, basolateral amygdala; CeA, central nucleus of the amygdala; CG, central gray; IL, infralimbic cortex; LH, lateral hypothalamus; PVN, paraventricular nucleus.
Acknowledgments
The authors thank Dr. Bruce Lyeth of University of California, Davis for his invaluable advice on fluid percussion injury. This study was supported by grants from the DOD (W81XWH-08-2-0134) and NIH (NS097149, NS086301).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bryant R.A., O'Donnell M.L., Creamer M., McFarlane A.C., Clark C.R., and Silove D. (2010). The psychiatric sequelae of traumatic injury. Am. J. Psychiatry 167, 312–320 [DOI] [PubMed] [Google Scholar]
- 2.Hibbard M.R., Ashman T.A., Spielman L.A., Chun D., Charatz H.J., and Melvin S. (2004). Relationship between depression and psychosocial functioning after traumatic brain injury. Arch. Phys. Med. Rehabil. 85, 4 Suppl. 2, S43–S53 [DOI] [PubMed] [Google Scholar]
- 3.Hibbard M.R., Uysal S., Kepler K., Bogdany J., and Silver J. (1998). Axis I psychopathology in individuals with traumatic brain injury. J. Head Trauma Rehabil. 13, 24–39 [DOI] [PubMed] [Google Scholar]
- 4.Bahraini N.H., Breshears R.E., Hernandez T.D., Schneider A.L., Forster J.E., and Brenner L.A. (2014). Traumatic brain injury and posttraumatic stress disorder. Psychiatr. Clin. North Am. 37, 55–75 [DOI] [PubMed] [Google Scholar]
- 5.Yurgil K.A., Barkauskas D.A., Vasterling J.J., Nievergelt C.M., Larson G.E., Schork N.J., Litz B.T., Nash W.P., and Baker D.G. (2014). Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry 71, 149–157 [DOI] [PubMed] [Google Scholar]
- 6.Maren S. and Holmes A. (2016). Stress and fear extinction. Neuropsychopharmacology 41, 58–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlov I.P. (1927). Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Ann. Neurosci. 17, 136–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goode T.D., and Maren S. (2014). Animal models of fear relapse. ILAR. J. 55, 246–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann D.L., and Longbottom P.L. (2008). The renewal of extinguished conditioned fear with fear-relevant and fear-irrelevant stimuli by a context change after extinction. Behav. Res.Ther. 46, 188–206 [DOI] [PubMed] [Google Scholar]
- 10.Maren S. (2014). Fear of the unexpected: hippocampus mediates novelty-induced return of extinguished fear in rats. Neurobiol. Learn. Mem. 108, 88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent V., and Westbrook R.F. (2009). Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn.Mem. 16, 520–529 [DOI] [PubMed] [Google Scholar]
- 12.Do-Monte F.H., Manzano-Nieves G., Quinones-Laracuente K., Ramos-Medina L., and Quirk G.J. (2015). Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J. Neurosci. 35, 3607–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milad M.R., and Quirk G.J. (2002). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74 [DOI] [PubMed] [Google Scholar]
- 14.Vidal-Gonzalez I., Vidal-Gonzalez B., Rauch S.L., and Quirk G.J. (2006). Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn. Mem. 13, 728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quirk G.J., and Mueller D. (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinard C.R., Mascagni F., and McDonald A.J. (2012). Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience 205, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon C.E., Lyeth B.G., Povlishock J.T., Findling R.L., Hamm R.J., Marmarou A., Young H.F., and Hayes R.L. (1987). A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 [DOI] [PubMed] [Google Scholar]
- 18.Kelley B.J., Farkas O., Lifshitz J., and Povlishock J.T. (2006). Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and Wallerian degeneration. Exp. Neurol. 198, 350–360 [DOI] [PubMed] [Google Scholar]
- 19.Floyd C.L., Golden K.M., Black R.T., Hamm R.J., and Lyeth B.G. (2002). Craniectomy position affects morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J. Neurotrauma. 19, 303–316 [DOI] [PubMed] [Google Scholar]
- 20.Hylin M.J., Orsi S.A., Zhao J., Bockhorst K., Perez A., Moore A.N., and Dash P.K. (2013). Behavioral and histopathological alterations resulting from mild fluid percussion injury. J. Neurotrauma. 30, 702–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coggeshall R.E., and Lekan H.A. (1996). Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J. Comp. Neurol. 364, 6–15 [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G., and Watson C. (2000). The Rat Brain in Stereotaxic Coordinates. Academic: San Diego, CA: [DOI] [PubMed] [Google Scholar]
- 23.Van De Werd H.J., Rajkowska G., Evers P., and Uylings H.B. (2010). Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct. Funct. 214, 339–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassell M.D., Chittick C.A., Siegel M.A., and Wright D.J. (1989). Collateralization of the amygdaloid projections of the rat prelimbic and infralimbic cortices. J. Comp. Neurol. 279, 235–248 [DOI] [PubMed] [Google Scholar]
- 25.Ferreira A.N., Yousuf H., Dalton S., and Sheets P.L. (2015). Highly differentiated cellular and circuit properties of infralimbic pyramidal neurons projecting to the periaqueductal gray and amygdala. Front. Cell. Neurosci. 9, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milad M.R., Vidal-Gonzalez I., and Quirk G.J. (2004). Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav. Neurosci. 118, 389–394 [DOI] [PubMed] [Google Scholar]
- 27.Sierra-Mercado D., Padilla-Coreano N., and Quirk G.J. (2011). Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald A.J., Shammah-Lagnado S.J., Shi C., and Davis M. (1999). Cortical afferents to the extended amygdala. Ann. N. Y. Acad. Sci. 877, 309–338 [DOI] [PubMed] [Google Scholar]
- 29.Knapska E., Macias M., Mikosz M., Nowak A., Owczarek D., Wawrzyniak M., Pieprzyk M., Cymerman I.A., Werka T., Sheng M., Maren S., Jaworski J., and Kaczmarek L. (2012). Functional anatomy of neural circuits regulating fear and extinction. Proc. Natl. Acad. Sci.U. S. A. 109, 17093–17098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herry C., Ciocchi S., Senn V., Demmou L., Muller C., and Luthi A. (2008). Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 [DOI] [PubMed] [Google Scholar]
- 31.Cho J.H., Deisseroth K., and Bolshakov V.Y. (2013). Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron 80, 1491–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orsini C.A., Kim J.H., Knapska E., and Maren S. (2011). Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J. Neurosci. 31, 17269–17277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eakin K., and Miller J.P. (2012). Mild traumatic brain injury is associated with impaired hippocampal spatiotemporal representation in the absence of histological changes. J. Neurotrauma 29, 1180–1187 [DOI] [PubMed] [Google Scholar]
- 34.Gurkoff G.G., Giza C.C., and Hovda D.A. (2006). Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 1077, 24–36 [DOI] [PubMed] [Google Scholar]
- 35.Reger M.L., Poulos A.M., Buen F., Giza C.C., Hovda D.A., and Fanselow M.S. (2012). Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol. Psychiatry. 71, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell J.N., Register D., and Churn S.B. (2012). Traumatic brain injury causes an FK506-sensitive loss and an overgrowth of dendritic spines in rat forebrain. J. Neurotrauma 29, 201–217 [DOI] [PubMed] [Google Scholar]
- 37.Campbell J.N., Gandhi A., Singh B., and Churn S.B. (2014). Traumatic brain injury causes a tacrolimus-sensitive increase in non-convulsive seizures in a rat model of post-traumatic epilepsy. Int. J. Neurol. Brain Disord. 1, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]