Figure 2.
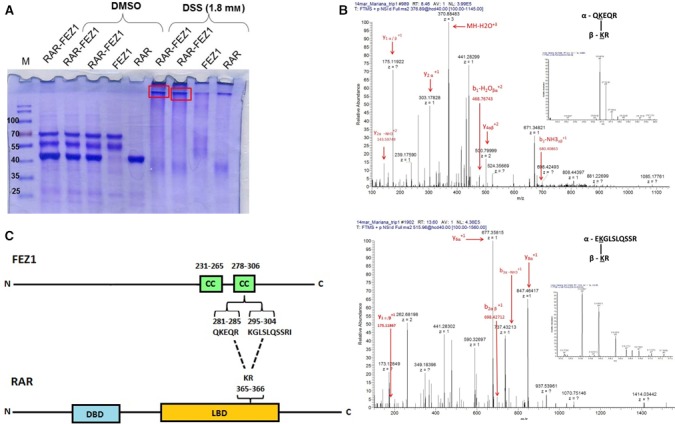
Binding interface of the FEZ1–RAR interaction. (A) Recombinant purified proteins were incubated with DSS and DMSO (control). Bands corresponding to complexes (red rectangles) were excised from polyacrylamide gel, digested with trypsin, and analyzed by LC‐MS/MS. (B) Spectra were manually validated for b and y ion series of the α (peptides of FEZ1) and β (peptides of RAR) chains. (C) Schematic representation of FEZ1 and RAR. FEZ1 presents two coiled‐coils (CC) predicted regions (231‐265 amino acids; 278‐306 amino acids). In RAR, the DBD (DNA binding domain) and LBD (ligand‐binding domain) are represented. Two predicted interaction sites were found for FEZ1, ranging from amino acids 281‐285 (QKEQR) and 295‐304 (KGLSLQSSRI). These two regions are in one of the FEZ1 coiled‐coil. The predicted binding region for RAR is located in its LBD, amino acids 365‐366 (KR). M = molecular mass marker. The indicated molecular masses of the marker protein bands are shown in kDa.
