Figure 6. A proposed model for the role of GLMN and IAPs in regulation of inflammasomes.
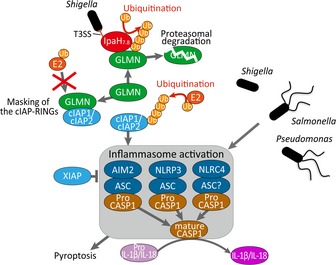
A schematic representation shows the machinery for the regulation of inflammasome activation caused by bacterial infection. In this study, we demonstrated that cIAP1 and cIAP2 are important for efficient inflammasome activation, whereas XIAP acts as a negative regulator of inflammasomes. Recent work showed that GLMN binds to small RING‐finger protein RBX1 (RING‐box1, an component of the Rbx1‐Cul1 E3 ligase), inhibiting its E3 ligase activity by masking the E2‐binding surface. In accordance with these observations, GLMN binds to the RING domains of cIAP1 and cIAP2, thereby inhibiting their functions. Shigella delivers the T3SS effector IpaH7.8, which targets GLMN at a specific stage of infection, thereby conferring an advantage on the bacterial invader. In our model, IpaH7.8 removes the masking GLMN from cIAPs via E3 ligase‐dependent proteolysis, leading to aberrant upregulation of inflammasomes.
