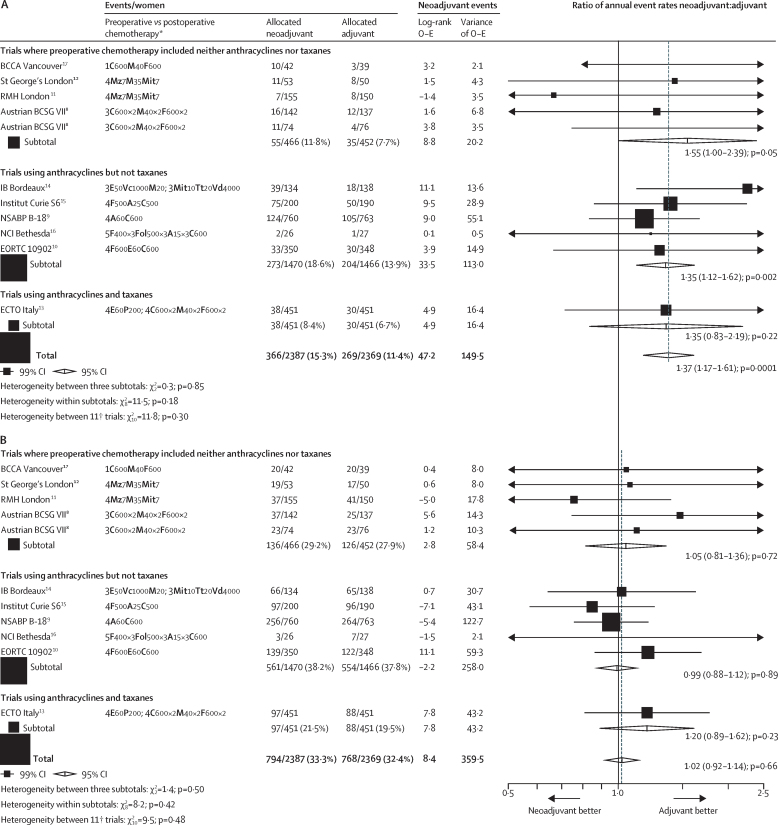
Figure 4.
Rate ratios for the effect of neoadjuvant versus adjuvant chemotherapy on recurrence by trial
(A) Local recurrence. (B) Distant recurrence. Three trials recorded causes of any deaths but only the first breast cancer event. Hence, for these trials, distant recurrence includes the first distant recurrence as the first event and death from breast cancer. The appendix (pp 3–4) contains a full description of each trial's chemotherapy regimen. A=doxorubicin (adriamycin). BCCA=British Columbia Cancer Agency. BCSG=Breast Cancer Study Group. C=cyclophosphamide. E=epirubicin. ECTO=European Cooperative Trial in Operable Breast Cancer. EORTC=European Organisation for Research and Treatment of Cancer. F=fluorouracil. Fol=folinic acid. IB=Institut Bergonié. M=methotrexate. Mit=mitomycin-C. Mz=mitoxantrone. NCI=National Cancer Institute. NSABP=National Surgical Adjuvant Breast and Bowel Project. O–E=observed minus expected. P=paclitaxel. Tt=thiotepa. Vc=vincristine. Vd=vindesine. *Chemotherapy regimens given preoperatively in those allocated neoadjuvant and postoperatively in those allocated adjuvant chemotherapy. The number of cycles, agents, and drug doses (in mg/m2) per cycle are given. †The Austrian BCSG VII trial8 has two entries to take into account the two postoperative chemotherapies given to both randomised groups (appendix pp 3–4).