Figure 2. Effects of mutations associated with CID–G/AI on the structure of the RAG complex.
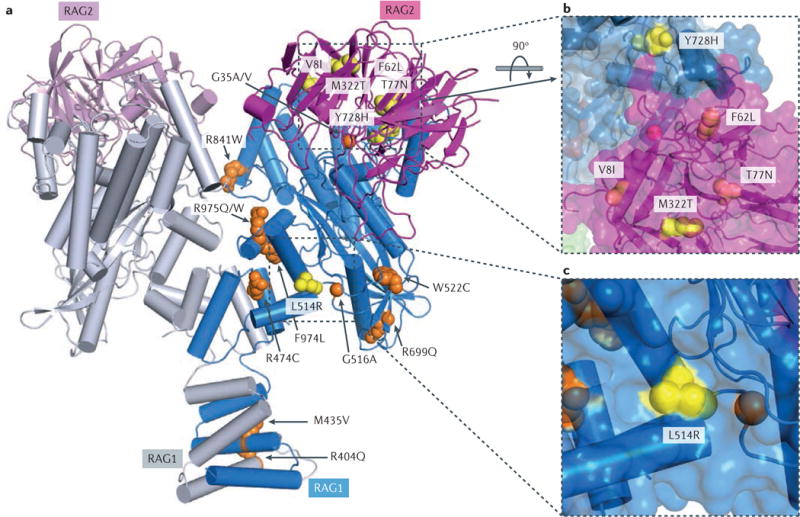
a| Structure of the recombination-activating gene 1 (RAG1)–RAG2 heterotetramer. Two RAG1 core subunits are shown in blue and grey, and two RAG2 core subunits are shown in purple and pink. Side chains of mutations found only in patients with combined immunodeficiency associated with granulomas and/or autoimmunity (CID–G/AI) are shown as yellow spheres, and those found in both patients with CID–G/AI and patients with severe combined immunodeficiency (SCID) or Omenn syndrome are shown in orange. For clarity, mutations are shown in one subunit of RAG1 and RAG2 only. All residues are numbered according to human RAG proteins. b,c | Zoom-in views of mutations that are unique to patients with CID–G/AI. Molecular surfaces are shown together with the ribbon diagram. L514R and Y728H from RAG1 and M322T from RAG2 are partially exposed (shown in yellow), whereas V8I, F62L and T77N from RAG2 are buried inside the protein (shown in purple). These mutations seem to lead to mild structural destabilization of the RAG proteins. The R841W mutation is at the interface of the closed conformation of the RAG complex.
