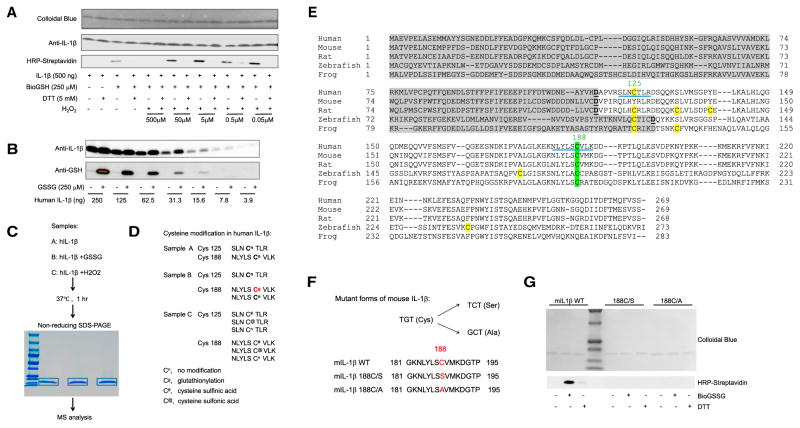
Figure 1. The Most Conserved Cysteine in Mature IL-1β Can Be S-Glutathionylated.
(A) Immunoblots of biotinylated glutathione (BioGSH)-modified human IL-1β with streptavidin-HRP. Total protein loading was evaluated with colloidal blue dye and IL-1β-specific antibodies.
(B) Immunoblots of S-glutathionylated human IL-1β using GSH-specific antibodies with total protein loading evaluated by IL-1β-specific antibodies.
(C) Graphical representation of the mass spectrometry (MS) analytical strategy for human IL-1β S-glutathionylation.
(D) Oxidation-induced cysteine modifications in human IL-1β detected by MS.
(E) Amino acid sequence alignments of full-length IL-1β. Gray highlight, IL-1β propiece; green highlight, conserved cysteines in mature IL-1β; yellow highlight, non-conserved cysteines in mature IL-1β; underline, cysteine-containing peptides detected in MS analysis.
(F) Amino acid sequences of WT and mutant forms of IL-1β.
(G) Immunoblots of S-glutathionylated WT and mutant forms of IL-1β using streptavidin-HRP. IL-1β was treated with oxidized biotinylated GSH BioGSSG (250 μM) in the presence or absence of DTT (5 mM). Total protein loading was evaluated by colloidal blue staining.