Figure 9. The MID-domain is required for both positive and negative functions of Doc2B in vesicle priming.
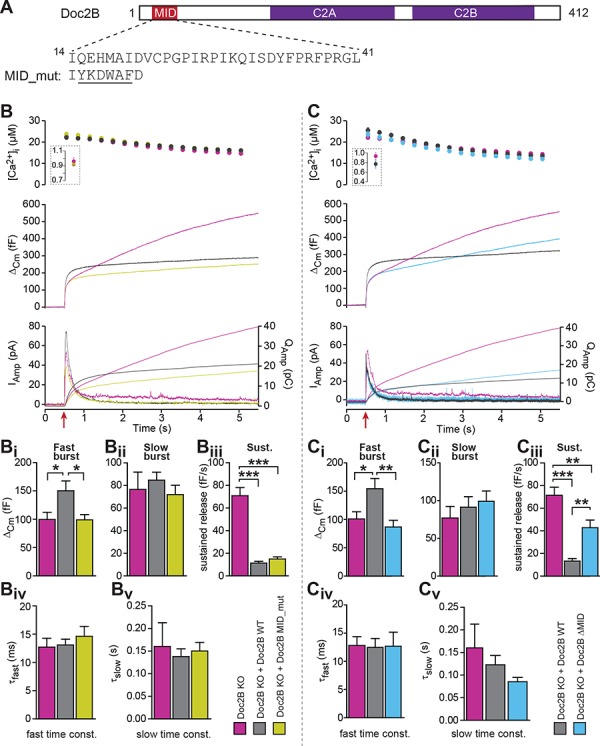
(A) Domain structure of mouse Doc2B. C2A and C2B are the two C2-domains. The MID-domain encompasses amino acids 14–38. We mutated the N-terminal part of the MID-domain (MID_mut, (Mochida et al., 1998)), or deleted it entirely (deletion of amino acids 14–41: ΔMID). (B) Ca2+ uncaging experiment in Doc2B KO cells (n = 25) and KO cells overexpressing Doc2B WT (n = 26) or Doc2B MID_mut (n = 26). (Bi-Bv) Kinetic analysis showed that mutating a stretch of the Doc2B MID domain abolishes the facilitating effect that Doc2B WT has in potentiating the RRP size (i.e. the size of the fast burst) (Bi) while causing a similar reduction in the rate of sustained release as the wildtype protein (Biii). (C), Full deletion of the Munc13-interacting domain of Doc2B (amino acids 14–41; Doc2B ΔMID) interferes with the function of Doc2B in both the fast burst and the sustained phase of release. Ca2+ uncaging experiment in Doc2B KO cells overexpressing Doc2B WT (n = 18) or Doc2B ΔMID (n = 20). (Ci-Cv) Kinetic analysis showing that removal of the full MID region of Doc2b interferes both with the promoting action on the filling of the fast pool of vesicles (Ci) and alleviates the suppression of the sustained phase of release caused by Doc2B WT overexpression (Ciii). The data from recordings of uninfected Doc2B KO cells used in panels C is the same as the one in panels B. *p<0.05, **p<0.01, ***p<0.001; Dunn’s multiple comparison test.
