Abstract
Rationale:
Primary osteosarcomas of the skull and skull base are rare, comprising <2% of all skull tumors. Primary osteosarcomas of the skull are aggressive neoplasms composed of spindle cells producing osteoid which have poor outcome.
Patient concerns:
A 33-year-old woman was admitted to our hospital with a major complaint of a growing mass on her left frontal region of the skull for 10 months. Prior to the accurate diagnosis, the mass on her skull was considered to be eosinophilic granuloma.
Diagnoses:
Computerized tomogram (CT) scan of skull revealed a lytic lesion causing destruction of left frontal bone with surrounding soft tissue mass. The histological examination of the lesion showed typical features of osteosarcoma.
Interventions:
The patient received 3 surgeries and adjuvant chemotherapy and radiotherapy for the frontal bone lesion.
Outcomes:
At the last follow-up, after 4 years, the patient was free of disease both clinically and on imaging by magnetic resonance imaging (MRI) scan after 4 years.
Lessons:
Because osteosarcoma of skull is a rare disease, the early recognition and correct diagnosis are very important for a better prognosis. It is therefore imperative that clinicians recognize osteosarcoma early to make an accurate diagnosis and complete surgical resection followed by combined chemo-radiation is proved to be one of the most optimal treatment regimens.
Keywords: chemo-radiation, frontal bone, primary osteosarcoma, surgical resection
1. Introduction
Osteosarcoma is the most common primary bone tumor which typically occurs in extremities.[1] However, osteosarcoma of skull is rare, comprising only 6% to 8% of osteosarcomas.[2] Skull osteosarcomas usually present in third to fourth decades of life,[3] which are secondary to treatment with chemotherapy or radiotherapy. They occur most frequently in the calvaria and then the skull base.[3,4] Among these osteosarcomas, primary osteosarcomas occur de novo and are less frequently encountered.[5] Herein, we report a case of osteosarcoma of the frontal bone and describe the radiological features, clinical symptoms, and the treatment outcome using surgical resection, adjuvant chemotherapy, and radiotherapy. Our study was approved by the Institutional Ethic Committee of The Third Xiangya Hospital, Central South University. Written informed consent has been provided by the patient to obtain her case details and any accompanying images published.
2. Case report
A 33-year-old woman was admitted to our hospital with a major complaint of a growing mass on her left frontal region of the skull for 10 months. The mass had been increasing in size for the past 10 months, causing the pain radiating all over the patient's left frontal of the head, gradually from the moderate and occasional type to the intense and continuous type. Her past medical history was unremarkable, and no similar cases were found in her family. Physical examination found a soft mass on the left frontal bone beneath the scalp, which showed tenderness upon palpation. There was neither sensory nor any cranial nerve deficit. Laboratory tests, including a blood routine test, were all normal. Computerized tomogram (CT) scan of skull revealed a lytic lesion causing destruction of left frontal bone with surrounding soft tissue mass with extension to the right frontal bone and the right frontal sinus (Fig. 1A). On magnetic resonance imaging (MRI), it was a well-circumscribed extra-axial mass measuring 5 × 7.5 × 4 cm which was hyperintense on T1-weighted and in T2-weighted series (Fig. 1B and C). CT of the chest and abdomen was normal. Because of the imaging results, differential diagnosis before surgery included chondromyxoid fibroma and chordoma. A bilateral frontal coronary craniotomy exposed the mass just under the scalp and bulging from the left frontal bone, associated with bony destruction, forming a cystic lesion. The mass was excised from the adjacent dura totally with a negative margin. Then a titanium mesh cranioplasty was performed over the calvarial defect. Histological examination of the operative specimen found proliferation of histiocyte-like cells in the lesion, which had irregularly contoured or folded nuclei. Infiltration of lymphocytes and neutrophils was also seen. The patient was diagnosed as eosinophilic granuloma. The patient recovered well after the surgery and was discharged on postoperative day 9 with no deficits. A CT scan performed 7 days postoperatively revealed the mass was excised (Fig. 1D).
Figure 1.
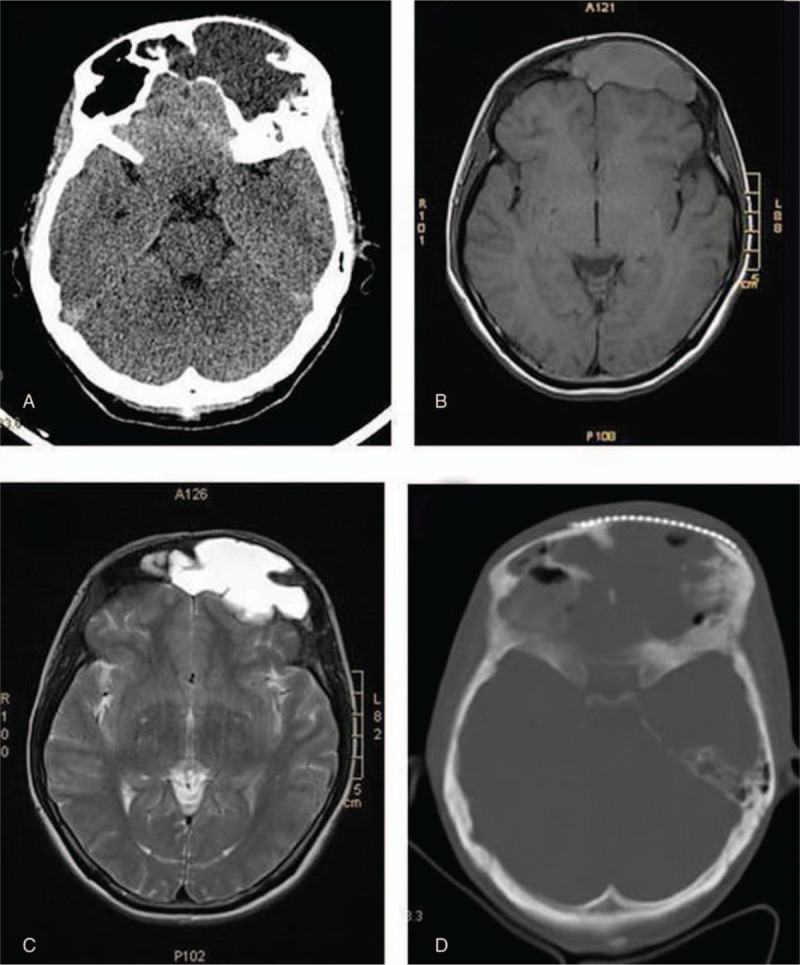
Preoperative computerized tomogram (CT) scan of skull revealed a lytic lesion causing destruction of left frontal bone with surrounding soft tissue mass with extension to the right frontal bone and the right frontal sinus (A); preoperative magnetic resonance imaging (MRI) showed a well-circumscribed extra-axial mass measuring 5 × 7.5 × 4 cm which was hyperintense on T1-weighted (B) and T2-weighted series (C); postoperative CT scan performed 7 days revealed the mass was excised (D).
The patient remained in good condition for about 18 months until an intracranial hypertension syndrome arose. CT revealed recurrence of tumor beneath the titanium mesh (Fig. 2A). Further surgical resection was performed to remove the tumor and the titanium mesh. The tumor was encapsulated without visible invasion of the underlying dura or overlying scalp. Postoperative CT scan revealed gross-total tumor excision, but it also showed contrast enhancement at the surgical dura and scalp, suggestive of residual tumor (Fig. 2B). Histopathologic analysis revealed proliferation of obviously malignant-appearing spindle-shaped cells, associated multinuclear giant cells and predominant osteoid matrix in the tumor (Fig. 3). Eventually, after consultation with several pathological experts, the final diagnosis of fibrohistiocytic osteosarcoma was confirmed. After surgery, the patient underwent adjuvant therapy in the form of both chemotherapy and radiotherapy. Initially, first cycle of chemotherapy for 7 days with Pirarubicin (30 mg) and Ifosfamide (2 g) were given. After first cycle of chemotherapy, the patient received 60 grays of adjuvant radiotherapy in 30 fractions over 4 weeks, followed again by 3 cycles of chemotherapy with Pirarubicin (30 mg) and Ifosfamide (2 g). Four months later, follow-up CT scan showed no recurrence of tumor. However, persistent dural enhancement was noted.
Figure 2.
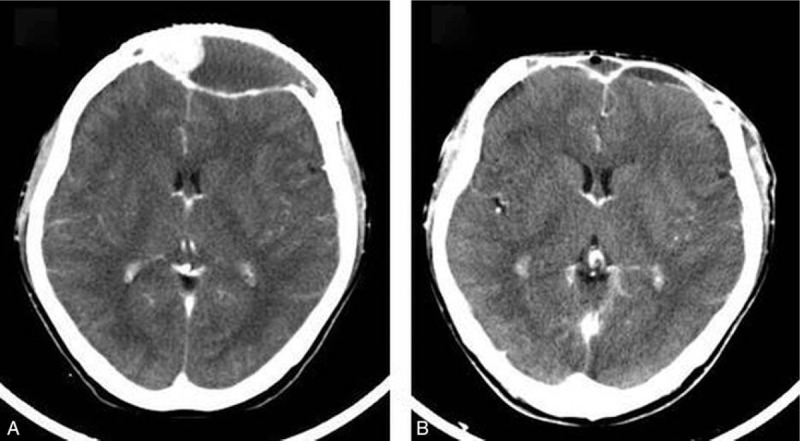
Enhanced CT scan of skull revealed recurrence of tumor beneath the titanium mesh (A); postoperative enhanced CT scan revealed gross-total tumor excision, but it also showed contrast enhancement at the surgical dura and scalp, suggestive of residual tumor (B). CT = computerized tomogram.
Figure 3.
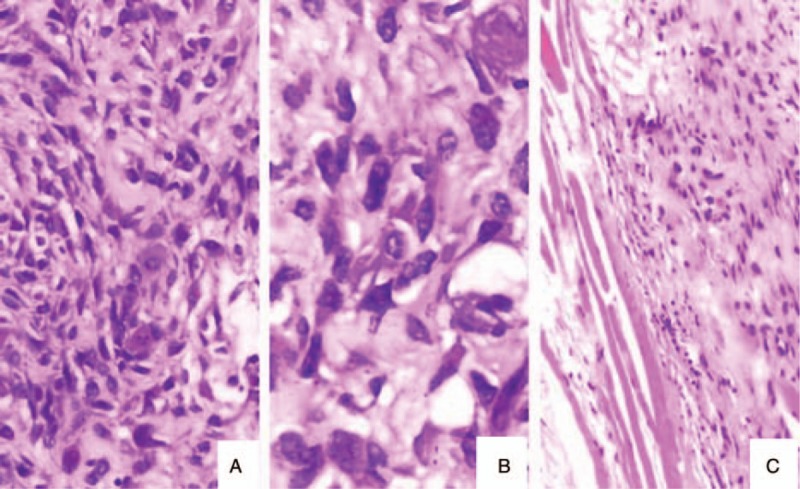
Histopathological examination showed proliferation of obviously malignant-appearing spindle-shaped cells, associated multinuclear giant cells (A: H&E ×200; B: H&E ×400) and predominant osteoid matrix in the tumor (C: H&E ×100).
After a disease-free interval of 2 years she developed a local recurrence of the tumor at the prior resection lesion. MRI revealed recurrence of tumor beneath the scalp and contrast enhancement at the surgical dura (Fig. 4A). Tc-99m methylene diphosphonate (MDP) skeletal scintigraphy showed no increasing uptake, suggesting no distant metastases. However, skull single photon emission computed tomography (SPECT) imaging demonstrated increasing uptake in the frontal region. Reoperation was undertaken, in which the tumor and the invaded dura matter were removed at the same location as previously and a duraplasty with self-tissue was performed to repair the dura mater defects. Histopathological examination of the excised specimen was consistent with osteosarcoma. Further chemotherapy for 7 days with Pirarubicin (30 mg) and Ifosfamide (2 g) was administered. At the last follow-up, the patient was free of disease both clinically and on imaging by MRI scan after 4 years (Fig. 4B).
Figure 4.
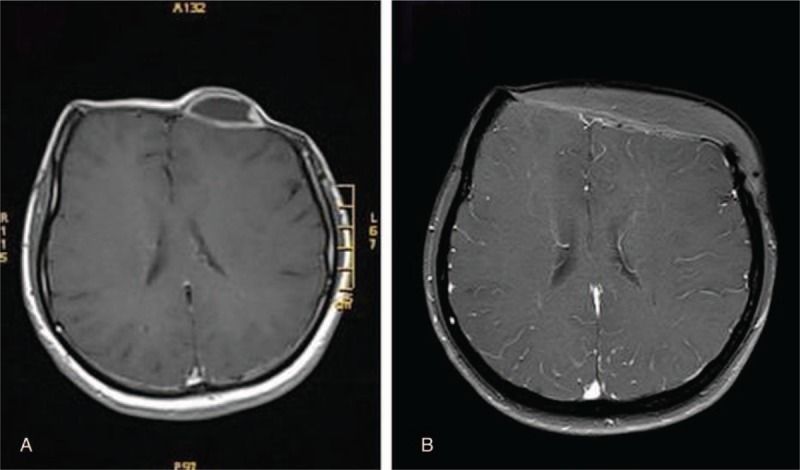
Enhanced MRI revealed recurrence of tumor beneath the scalp and contrast enhancement at the surgical dura (A); enhanced MRI done 4 years after surgery showed no recurrence of tumor (B). MRI = magnetic resonance imaging.
3. Discussion
Primary osteosarcoma of the skull is rare, with an incidence of approximately 1% to 2% of all skull tumors. Only fewer than 150 cases have been reported.[6–18] The occurrence of osteosarcoma in the skull peaks in the third decade.[15,17]
The etiology of osteosarcoma is still uncertain, but the major risk for development of skull osteosarcoma is similar to those for osteosarcoma of the long skeletal bones, consisting of radiation exposure, Paget disease, Li-Fraumeni syndrome, and retinoblastoma.[15,19–21] The skull is a favored site for osteosarcoma arising out of Paget disease, with a higher incidence of Paget disease-associated osteosarcomas than of primary osteosarcomas at this site.[7,19] Other bone abnormalities, such as fibrous dysplasia, multiple osteochondromatosis, chronic osteomyelitis, myositis ossificans, and trauma have also been proposed as risk factors.[19]
The clinical symptoms of primary skull osteosarcoma vary depending on the tumor site.[7] Like osteosarcoma of the extremities, skull osteosarcomas frequently present as a slow-growing mass or swelling. Unlike extremity tumors, however, skull osteosarcomas are often painless or only of mildly pain.[7,19,22] Patients with skull osteosarcoma frequently present with headache, cranial nerve palsies, exophthalmos, visual impairment, or cranial hypertension.[7,23]
While osteosarcoma of extremities shows early metastasis, particularly to the lungs or brain, metastasis of skull osteosarcoma is uncommon.[24] However, relapse of skull osteosarcoma most often occurs locally.[25]
Osteosarcomas are spindle-cell tumors associated with excessive production of irregular and immature bones.[19] Osteosarcomas can be classified into several subtypes based on histological appearance. The most common subtypes include osteoblastic, chondroblastic, and fibroblastic osteosarcomas.[26] Other less common histological subtypes include telangiectatic, parosteal, periosteal, and small cell osteosarcomas.[14] The grading of osteosarcoma which can be classified as low, intermediate, or high grade is based on the degree of cellular atypia and architectural distortion.[5,27] In our case, the patient had a high-grade lesion. However, there is no significant correlation between the histologic character of the tumor and the prognosis.[4,20,21]
CT scan with bone windows plays the key role in diagnosis, in which bone growth with lytic regions and periosteal remodeling are the most common imaging features.[4,7,28] Contrast enhanced MRI is especially useful for assessing soft tissue involvement. High-grade osteosarcomas in MRI are usually isointense on T1-weighted, hypointense on T2-weighted, and enhance homogenously with well-defined margins on contrast images.[29] A chest CT scan and a radionuclide bone scan can be used to evaluate the existence of lung and skeletal metastases, respectively.[30,31]
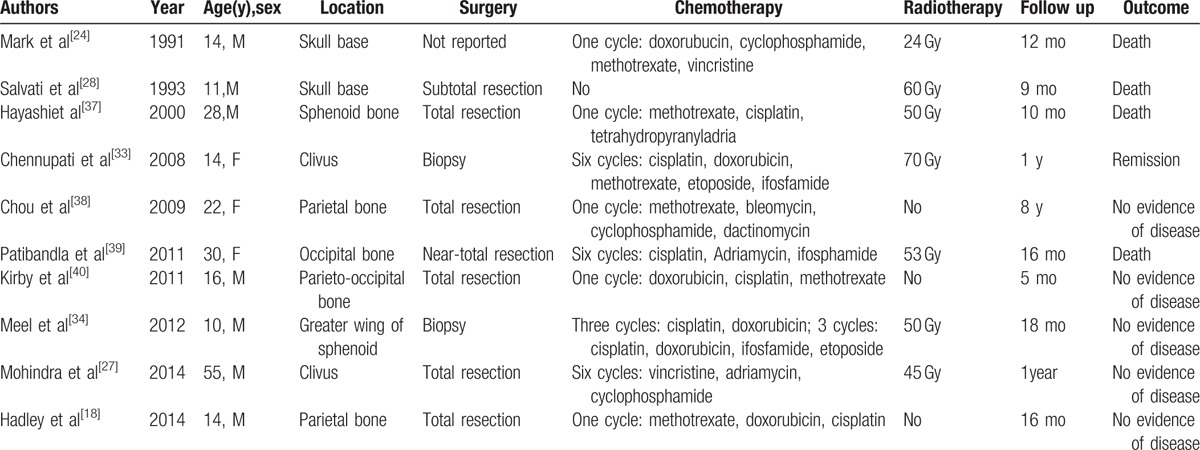
Osteosarcoma of skull is rare, causing the few evidence of optimal management strategy. Although, the methods of treatment are widely varied, surgical resection is still the mainstream.[32] Complete surgical excision and wide surgical margins have been associated with improved survival.[14,15,19] In our case, the invaded dura matter was excised during the third surgery to prevent recurrence. Because dura enhancement may represent tumor invasion, excision of involved dura is an important component of achieving maximal resection.[18] Because of the anatomy of the head, complete resection may be difficult to achieve. Furthermore, an aggressive surgical approach can cause a significant functional impairment or cosmetic defect.[13] Thus, local recurrence is the main reason of treatment failure and decrease 5-year survival rates in skull osteosarcomas.[33] Because complete surgical excision is difficult to achieve and skull osteosarcomas are likely to have positive tumor margins, adjuvant therapy is needed in most of cases.[34] Some studies have reported chemotherapy increased 5-year survival rates for patients with localized tumors from 20% to 60–70%.[7,27,33,35,36] Based on our review of the 10 case reports on PubMed (Table 1), the most commonly used agents are doxorubicin, cisplatin, methotrexate, and ifosfamide.[14,15,19,29,31,33] Chemotherapy may improve outcomes by decreasing the tumor to improve a better chance of total surgical resection or managing residual tumor when gross total resection is not achievable.[41] For subtotal or uncertain resection, radiation therapy has been shown to improve outcome. However, for patients with negative resection margins, radiotherapy did not improve survival.[42] The radiation dose administered in the past varies from 30 to 70 Gy.[27] Recently, recommended treatment includes: surgery aiming at complete resection; adjuvant chemotherapy when either complete resection is not attainable or lesions are of high grade; adjuvant radiotherapy when only subtotal or uncertain resection is achieved.[35]
Table 1.
Clinical review of 10 previously published primary osteosarcoma of skull.
4. Conclusion
As osteosarcoma of skull is a rare disease, correct diagnosis and proper treatment plans are difficult. Therefore, the uncommon entity needs to be considered when skull lesions demonstrate. Complete surgical resection followed by combined chemo-radiation is proved to be one of the most optimal treatment regimens, which can be considered as a primary treatment option.
Footnotes
Abbreviations: CT = computerized tomogram, MDP = methylene diphosphonate, MRI = magnetic resonance imaging, SPECT = single photon emission computed tomography.
Informed consent: Informed consent was obtained from all individual participants included in the study.
This study was funded by the National Natural Science Foundation of China (81602211).
The authors declare that they have no conflict of interest.
References
- [1].Anil S, Krishnan AP, Rajendran R. Osteosarcoma of the mandible masquerading as a dental abscess: report of a case. Case Rep Dent 2012;2012:635062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Caron AS, Hajdu SI, Strong EW. Osteogenic sarcoma of the facial and cranial bones. A review of forty-three cases. Am J Surg 1971;122:719–25. [DOI] [PubMed] [Google Scholar]
- [3].Garrington GE, Scofield HH, Cornyn J, et al. Osteosarcoma of the jaws. Analysis of 56 cases. Cancer 1967;20:377–91. [DOI] [PubMed] [Google Scholar]
- [4].Vege DS, Borges AM, Aggrawal K, et al. Osteosarcoma of the craniofacial bones. A clinico-pathological study. J Craniomaxillofac Surg 1991;19:90–3. [DOI] [PubMed] [Google Scholar]
- [5].Mathkour M, Garces J, Beard B, et al. Primary high-grade osteosarcoma of the clivus: a case report and literature review. World Neurosurg 2016;89:730.e9–13. [DOI] [PubMed] [Google Scholar]
- [6].Nora FE, Unni KK, Pritchard DJ, et al. Osteosarcoma of extragnathic craniofacial bones. Mayo Clin Proc 1983;58:268–72. [PubMed] [Google Scholar]
- [7].Salvati M, Ciappetta P, Raco A. Osteosarcomas of the skull. Clinical remarks on 19 cases. Cancer 1993;71:2210–6. [DOI] [PubMed] [Google Scholar]
- [8].Huvos AG, Sundaresan N, Bretsky SS, et al. Osteogenic sarcoma of the skull. A clinicopathologic study of 19 patients. Cancer 1985;56:1214–21. [DOI] [PubMed] [Google Scholar]
- [9].Bose B. Primary osteogenic sarcoma of the skull. Surg Neurol 2002;58:234–9. discussion 239-40. [DOI] [PubMed] [Google Scholar]
- [10].Abe K, Kosuda S, Kusano S, et al. Detection of recurrent skull osteosarcoma by skull single photon emission computed tomography using Tc-99m methylene diphosphonate. Clin Nucl Med 2004;29:72–3. [DOI] [PubMed] [Google Scholar]
- [11].Ashkan K, Pollock J, D’Arrigo C, et al. Intracranial osteosarcomas: report of four cases and review of the literature. J Neurooncol 1998;40:87–96. [DOI] [PubMed] [Google Scholar]
- [12].Chang CS, Bergeron L, Liao CC, et al. Craniofacial reconstruction of primary osteogenic sarcoma of the skull. J Plast Reconstr Aesthet Surg 2010;63:1265–8. [DOI] [PubMed] [Google Scholar]
- [13].Daw NC, Mahmoud HH, Meyer WH, et al. Bone sarcomas of the head and neck in children: the St Jude Children's Research Hospital experience. Cancer 2000;88:2172–80. [DOI] [PubMed] [Google Scholar]
- [14].Gadwal SR, Gannon FH, Fanburg-Smith JC, et al. Primary osteosarcoma of the head and neck in pediatric patients: a clinicopathologic study of 22 cases with a review of the literature. Cancer 2001;91:598–605. [PubMed] [Google Scholar]
- [15].Ha PK, Eisele DW, Frassica FJ, et al. Osteosarcoma of the head and neck: a review of the Johns Hopkins experience. Laryngoscope 1999;109:964–9. [DOI] [PubMed] [Google Scholar]
- [16].Jasnau S, Meyer U, Potratz J, et al. Craniofacial osteosarcoma experience of the cooperative German-Austrian-Swiss osteosarcoma study group. Oral Oncol 2008;44:286–94. [DOI] [PubMed] [Google Scholar]
- [17].Kanazawa R, Yoshida D, Takahashi H, et al. Osteosarcoma arising from the skull—case report. Neurol Med Chir (Tokyo) 2003;43:88–91. [DOI] [PubMed] [Google Scholar]
- [18].Hadley C, Gressot LV, Patel AJ, et al. Osteosarcoma of the cranial vault and skull base in pediatric patients. J Neurosurg Pediatr 2014;13:380–7. [DOI] [PubMed] [Google Scholar]
- [19].Oda D, Bavisotto LM, Schmidt RA, et al. Head and neck osteosarcoma at the University of Washington. Head Neck 1997;19:513–23. [DOI] [PubMed] [Google Scholar]
- [20].Vener J, Rice DH, Newman AN. Osteosarcoma and chondrosarcoma of the head and neck. Laryngoscope 1984;94(2 Pt 1):240–2. [DOI] [PubMed] [Google Scholar]
- [21].Zorzan G, Tullio A, Bertolini F, et al. Osteosarcoma of the mandibular condyle: case report. J Oral Maxillofac Surg 2001;59:574–7. [DOI] [PubMed] [Google Scholar]
- [22].Haque F, Fazal ST, Ahmad SA, et al. Primary osteosarcoma of the skull. Australas Radiol 2006;50:63–5. [DOI] [PubMed] [Google Scholar]
- [23].Byrne J, Fears TR, Whitney C, et al. Survival after retinoblastoma: long-term consequences and family history of cancer. Med Pediatr Oncol 1995;24:160–5. [DOI] [PubMed] [Google Scholar]
- [24].Mark RJ, Sercarz JA, Tran L, et al. Osteogenic sarcoma of the head and neck. The UCLA experience. Arch Otolaryngol Head Neck Surg 1991;117:761–6. [DOI] [PubMed] [Google Scholar]
- [25].Batsakis JG, Solomon AR, Rice DH. The pathology of head and neck tumors: neoplasms of cartilage, bone, and the notochord, part 7. Head Neck Surg 1980;3:43–57. [DOI] [PubMed] [Google Scholar]
- [26].Inwards CY, Unni KK. Classification and grading of bone sarcomas. Hematol Oncol Clin North Am 1995;9:545–69. [PubMed] [Google Scholar]
- [27].Mohindra S, Savardekar A, Mahalingam SS, et al. Primary osteosarcoma of clivus: a short report. Br J Neurosurg 2014;28:531–3. [DOI] [PubMed] [Google Scholar]
- [28].Salvati M, Ciappetta P, Capone R, et al. Osteosarcoma of the skull in a child: case report and review of the literature. Childs Nerv Syst 1993;9:437–9. [DOI] [PubMed] [Google Scholar]
- [29].Geetha N, Kumar A, Ramachandran K, et al. Osteosarcoma of the sella. Australas Radiol 1999;43:517–9. [DOI] [PubMed] [Google Scholar]
- [30].Mascarenhas L, Peteiro A, Ribeiro CA, et al. Skull osteosarcoma: illustrated review. Acta Neurochir (Wien) 2004;146:1235–9. [DOI] [PubMed] [Google Scholar]
- [31].Marina N, Gebhardt M, Teot L, et al. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist 2004;9:422–41. [DOI] [PubMed] [Google Scholar]
- [32].Federman N, Bernthal N, Eilber FC, et al. The multidisciplinary management of osteosarcoma. Curr Treat Options Oncol 2009;10:82–93. [DOI] [PubMed] [Google Scholar]
- [33].Chennupati SK, Norris R, Dunham B, et al. Osteosarcoma of the skull base: case report and review of literature. Int J Pediatr Otorhinolaryngol 2008;72:115–9. [DOI] [PubMed] [Google Scholar]
- [34].Meel R, Thulkar S, Sharma MC, et al. Childhood osteosarcoma of greater wing of sphenoid: case report and review of literature. J Pediatr Hematol Oncol 2012;34:e59–62. [DOI] [PubMed] [Google Scholar]
- [35].Patel SG, Meyers P, Huvos AG, et al. Improved outcomes in patients with osteogenic sarcoma of the head and neck. Cancer 2002;95:1495–503. [DOI] [PubMed] [Google Scholar]
- [36].Smith RB, Apostolakis LW, Karnell LH, et al. National cancer data base report on osteosarcoma of the head and neck. Cancer 2003;98:1670–80. [DOI] [PubMed] [Google Scholar]
- [37].Hayashi T, Kuroshima Y, Yoshida K, et al. Primary osteosarcoma of the sphenoid bone with extensive periosteal extension—case report. Neurol Med Chir (Tokyo) 2000;40:419–22. [DOI] [PubMed] [Google Scholar]
- [38].Chou EK, Chang CS, Chen PK, et al. Long-term management of craniofacial osteosarcoma. J Craniofac Surg 2009;20:406–9. [DOI] [PubMed] [Google Scholar]
- [39].Patibandla MR, Uppin SG, Thotakura AK, et al. Primary telangiectatic osteosarcoma of occipital bone: a case report and review of literature. Neurol India 2011;59:117–9. [DOI] [PubMed] [Google Scholar]
- [40].Kirby EJ, Zhou HH, Morales L., Jr Primary pediatric osteosarcoma of the skull. J Craniofac Surg 2011;22:2399–405. [DOI] [PubMed] [Google Scholar]
- [41].Smeele LE, Kostense PJ, van der Waal I, et al. Effect of chemotherapy on survival of craniofacial osteosarcoma: a systematic review of 201 patients. J Clin Oncol 1997;15:363–7. [DOI] [PubMed] [Google Scholar]
- [42].Guadagnolo BA, Zagars GK, Raymond AK, et al. Osteosarcoma of the jaw/craniofacial region: outcomes after multimodality treatment. Cancer 2009;115:3262–70. [DOI] [PubMed] [Google Scholar]


