Figure 6.
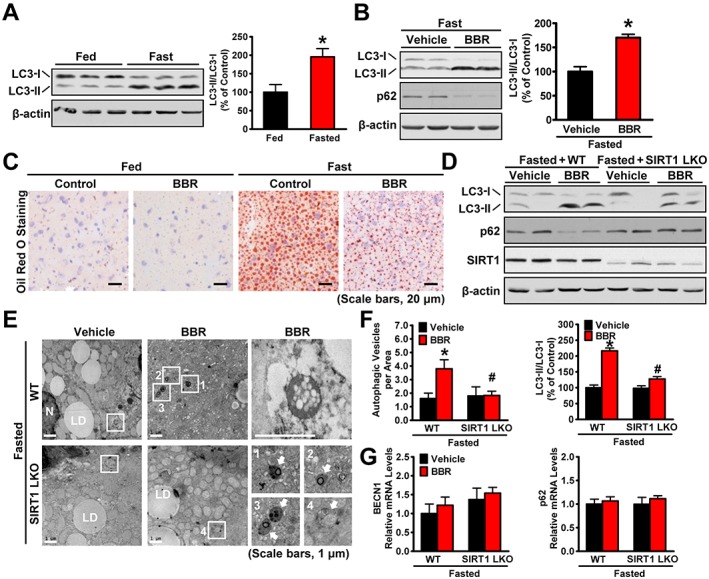
SIRT1 is required for berberine (BBR) to potentiate fasting‐induced autophagy in mouse livers. Male C57BL/6 mice at 8 weeks of age and SIRT1 LKO or WT mice at 16 weeks of age were treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) by i.p. injection once daily for 4 weeks and then subjected to feeding (fed) or fasting for 24 h (fasted). (A) The conversion of LC3‐I to LC3‐II was increased by fasting in mice. Band intensity of LC3‐II was quantified by densitometry and normalized to the levels of LC3‐I for unwanted sources of variation and presented as the mean ± SEM, n = 5. *P < 0.05, versus fed mice. (B) Berberine stimulates the conversion of LC3‐I to LC3‐II and reduces the p62 in mice in response to fasting, n = 5. (C) Hepatic steatosis was assessed by Oil Red O staining (scale bars: 50 μm). (D) SIRT1 deficiency attenuated berberine‐induced conversion of LC3‐I to LC3‐II and the reduction of p62 in the liver of fasted mice, n = 6. (E) Electron micrographs and the (F) average numbers of autophagic vacuoles (AVs) in the liver tissue were measured. Images 1–4 showing AVs are high magnifications of the ‘white box’ area indicated in the images of berberine‐treated WT or SIRT1 LKO mice in response to fasting. Arrows denote AVs. N, nucleus; LD, lipid droplet. The average numbers of AVs were quantified from a randomly selected pool of five fields under each condition. The data are presented as the mean ± SEM, n = 5. *P < 0.05, versus WT and vehicle; #P < 0.05, versus WT and berberine. (G) The mRNA levels of BECN1 and p62 were measured by real‐time PCR. The data were normalized to β‐actin for unwanted sources of variation and presented as the mean ± SEM, n = 5.
