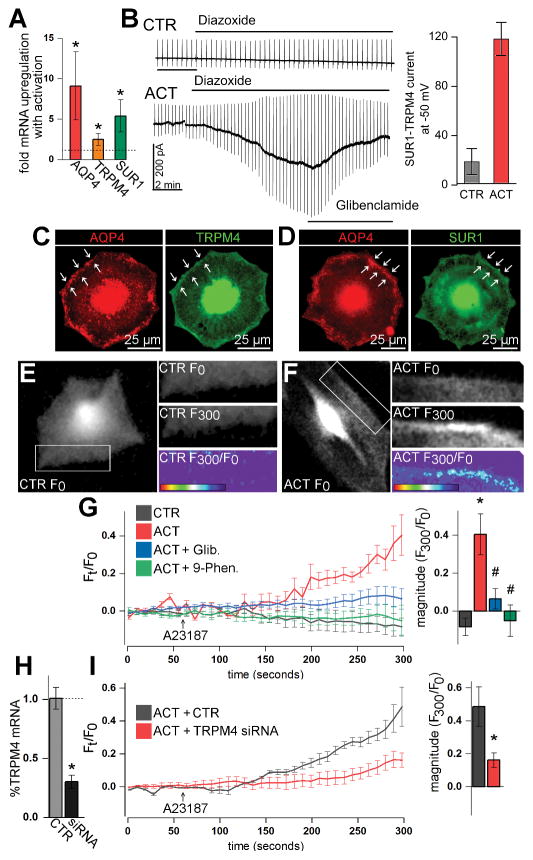
Figure 6. The SUR1-TRPM4-AQP4 complex mediates swelling of activated primary astrocytes.
(A, B) Activation (ACT) of primary mouse astrocytes with overnight TNFα, IFNγ, and LPS (20 ng/mL, 20 ng/mL, 1 μg/mL, respectively) resulted in upregulation of AQP4, TRPM4, and SUR1 mRNA versus control (CTR) astrocytes (A), and expression of functional SUR1-TRPM4 channels that mediate inward glibenclamide-sensitive current upon exposure to the SUR1 activator, diazoxide (100 μM) (B); *p<0.05 in t-test versus control; n=5 replicates for qPCR and n>15 cells per condition for electrophysiology. (C, D) Fluorescence immunocytochemistry micrographs of activated astrocytes showing that AQP4 closely co-localizes with TRPM4 (C) and SUR1 (D) on the plasmalemma cell margin (arrows). (E, F) Calcein fluorescence micrographs of astrocytes at time 0 seconds (F0) and at time 300 seconds (F300) in experiments with A23187 calcium ionophore, showing increased membrane calcein fluorescence in activated, but not in control astrocytes upon A23187 application; F300/F0 heat maps show change in calcein fluorescence with A23187 treatment. (G) Traces of calcein fluorescence (Ft/F0) in primary astrocytes showing that, compared to control astrocytes (grey), activation (red) sensitizes astrocytes to A23187-induced water influx, which is inhibited with glibenclamide (Glib.) (blue) and blocked with 9-phenanthrol (9-Phen.) (green). (H, I) Compared to control (CTR), ~70% knockdown of TRPM4 mRNA with siRNA (H) resulted in ~65% reduction in activated astrocyte swelling upon A23187 application; n=3 independent experiments with 3 cells/experiment; *p<0.05 in t-test or ANOVA with Tukey tests versus leftmost column; #p<0.05 in ANOVA with Tukey tests versus ACT.