Abstract
A hallmark behavioral feature of fragile X syndrome (FXS) is the propensity for individuals with the syndrome to exhibit significant impairments in social gaze during interactions with others. However, previous studies employing eye tracking methodology to investigate this phenomenon have been limited to presenting static photographs or videos of social interactions rather than employing a real-life social partner. To improve upon previous studies, we used a customized eye tracking configuration to quantify the social gaze of 51 individuals with FXS and 19 controls, aged 14–28 years, while they engaged in a naturalistic face-to-face social interaction with a female experimenter. Importantly, our control group was matched to the FXS group on age, developmental functioning, and degree of autistic symptomatology. Results showed that participants with FXS spent significantly less time looking at the face and had shorter episodes (and longer inter-episodes) of social gaze than controls. Regression analyses indicated that communication ability predicted higher levels of social gaze in individuals with FXS, but not in controls. Conversely, degree of autistic symptoms predicted lower levels of social gaze in controls, but not in individuals with FXS. Taken together, these data indicate that naturalistic social gaze in FXS can be measured objectively using existing eye tracking technology during face-to-face social interactions. Given that impairments in social gaze were specific to FXS, this paradigm could be employed as an objective and ecologically valid outcome measure in ongoing Phase II/Phase III clinical trials of FXS-specific interventions.
Keywords: eye tracking, social gaze, autism, fragile X syndrome
INTRODUCTION
Children diagnosed with genetic syndromes associated with intellectual and developmental disability (e.g., fragile X syndrome, Williams syndrome) often engage in highly specific forms of aberrant social behavior that can interfere with everyday functioning. For example, individuals diagnosed with Williams syndrome show a particular form of hypersociability in which they actively seek out social interactions with others [Jones et al., 2000; Frigerio et al., 2006]. Conversely, children with fragile X syndrome (FXS) commonly show deficits in social gaze behavior in which interactions with others are actively avoided [Cohen et al., 1988; Cohen et al., 1989; Cohen et al., 1991; Hall et al., 2006; Hall et al., 2009]. These contrasting behavioral phenotypes have been considered useful and important models for investigations examining the interplay between genes and environment [Kennedy et al., 2001; Schroeder et al., 2001].
FXS is a particularly interesting model of potential gene-environment interactions because it is a “single-gene” disorder. The disease affects approximately 1 in 3,000 individuals in the United States (approx. 100,000 people) and is the most common known form of inherited intellectual disability [Hagerman, 2008]. First described by Martin and Bell in 1943 as a “pedigree of mental defect showing sex linkage” [Martin and Bell, 1943], FXS is caused by mutations to the FMR1 gene at locus 27.3 on the long arm of the X chromosome [Verkerk et al., 1991]. Excessive methylation of the gene results in reduced or absent Fragile X Mental Retardation Protein (FMRP), a key protein involved in synaptic plasticity and dendritic maturation in the brain [Greenough et al., 2001; Soden and Chen, 2010]. As a consequence, individuals with FXS exhibit a cascade of developmental and cognitive deficits [Reiss and Dant, 2003] including impairments in executive functioning, visual memory, and perception, mental manipulation of visual-spatial relationships among objects, aberrant processing of arithmetical stimuli, as well as increased risk for autistic-like behaviors (e.g., social avoidance, communication impairments, and repetitive behaviors) [Sudhalter et al., 1990; Bennetto et al., 2001; Mazzocco, 2001; Cornish et al., 2004; Kaufmann et al., 2004; Skinner et al., 2005; Hall et al., 2006; Mazzocco et al., 2006; Sullivan et al., 2006; Sullivan et al., 2007; Dissanayake et al., 2009; Hall et al., 2009; Murphy, 2009]. FXS has therefore increasingly, but perhaps incorrectly, been considered a genetic model of autism itself [Hall et al., 2009].
Social gaze avoidance during interactions with others is a particularly prominent behavioral feature of individuals with FXS [Cohen et al., 1988]. For example, during a standardized 20-min face-to-face conversation with an unfamiliar research assistant, Hall and colleagues showed that boys with FXS engaged in social gaze avoidance approximately 90% of the time, and exhibited high levels of physiological arousal (as evidenced by elevated heart rate and low vagal tone) [Hall et al., 2009]. By contrast, age-matched typically developing siblings engaged in these behaviors for only 10% of the time and experienced significantly lower levels of physiological arousal. On the basis of these studies and others, several authors have suggested that social impairments observed in children with FXS may be caused by different underlying mechanisms than those diagnosed with Autism Spectrum Disorder (ASD) [Cohen et al., 1988; Cohen et al., 1989; Cohen et al., 1991; Cornish et al., 2007b; Cornish et al., 2008; Hall et al., 2010]. For example, given that children with FXS are able to discriminate between familiar and unfamiliar persons, and exhibit symptoms of social anxiety during social encounters with unfamiliar people [Cohen et al., 1988; Cohen et al., 1989; Cohen et al., 1991; Hall et al., 2009], it has been suggested that social impairments may be related to social anxiety and hyperarousal in FXS, whereas in ASD they may be related to social indifference [Cohen et al., 1988; Cornish et al., 2008].
Maintaining appropriate social gaze is a critical prerequisite for language development, emotion recognition, social engagement, and general learning through joint attention [Emery, 2000; Morales et al., 2000; Farroni et al., 2002; Brooks and Meltzoff, 2005; Csibra and Gergely, 2006; Itier and Batty, 2009; Senju and Johnson, 2009]. For example, several studies have indicated that high levels of social gaze avoidance can negatively impact social interaction skills and communication flow, given that crucial non-verbal gestures and facial expressions that usually aid social interaction will be missed [Doherty-Sneddon et al., 2013; Riby et al., 2012]. Given these factors, it seems important to examine and quantify social gaze in FXS in a naturalistic social setting (i.e., while individuals are actually engaged in a “real-life” social interaction).
Surprisingly few studies have attempted to employ eye tracking methodology to quantify social gaze during a real-life social setting. Part of the problem concerns the difficulty of setting up an experimental situation in which eye tracking data can be collected simultaneously while the person is engaged in a conversation with a “live” person. Studies conducted to date have therefore been limited to showing static photographs of faces or videos to individuals with FXS and quantifying which areas of the face are most commonly viewed [Cornish et al., 2007a; Dalton et al., 2008; Holsen et al., 2008; Shaw and Porter, 2013; Williams et al., 2013]. In a study conducted by Farzin et al. [2009], for example, photographs of faces that depicted various emotions were presented to 16 individuals with FXS (13 male, 3 female) and 16 typically developing controls. These authors found that participants with FXS looked significantly less at the eye region of the faces, and were more likely to look at the nose region compared to age-matched typically developing individuals. These findings were largely replicated in a second study by Farzin et al. [2011] in which 15 individuals with FXS and 20 age-matched typically developing controls were required to view photographs of the faces in two separate sessions. As before, these authors found that participants with FXS engaged in significantly less eye gaze than typically developing controls. However, in that study, participants with FXS were more likely to look at the mouth region of the face and no associations were found between levels of eye gaze and degree of autistic symptomatology. Limitations of both studies include the small number of individuals studied, the fact that no social interaction was actually presented, and that the controls were not matched on level of functioning or autistic symptomatology to the participants with FXS. In the present study, we therefore recruited a larger sample of individuals with FXS (males and females) and improved the ecological validity of the experimental setting by employing a real-life social partner (a female research assistant) who interacted directly with the participant during the experiment. In order to control for the confounds of developmental functioning and degree of autistic symptomatology on levels of social gaze, we compared the levels of social gaze in FXS to those observed in a group of individuals who had similar levels of developmental functioning and autistic symptomatology, but who did not have FXS or any other known genetic syndrome. We also examined potential differences between males and females with FXS. However, this latter comparison was of less interest to us given that, as a group, males with FXS are already known to be significantly more impaired than females with respect to intellectual ability and behavior.
We had four questions: (1) Can social gaze in FXS be measured in a naturalistic social setting using eye tracking methodology? (2) Which areas of the face do individuals with FXS look at when they are engaged in social interaction with an unfamiliar person? (3) How does social gaze behavior in FXS differ from social gaze behavior shown by age- and developmental age-matched individuals? (4) What effect does age, communication ability and degree of autistic symptoms have on the proportion of social gaze observed?
MATERIALS AND METHODS
Participants
Participants were recruited for the present study over a 3-year period as part of a longitudinal investigation of brain function and development conducted at Stanford University. Individuals with FXS were included in the study if they were aged between 12 and 28 years inclusive, had a genetic diagnosis of FXS (full mutation with evidence of aberrant methylation of the FMR1 gene, confirmed by genetic testing), were able to travel to Stanford for a 3-day visit and could remain seated in a chair during a social interaction for approximately 10 min without attempting to leave the room. Individuals in the control group were recruited from the local area (e.g., Regional centers, special education centers) and were included if they were aged between 12 and 28 years, and had been diagnosed with an unspecified form of developmental disability (i.e., not FXS or other known genetic disorder associated with intellectual disability such as Down syndrome, Prader-Willi syndrome, Turner syndrome, or Williams syndrome). Control participants were subsequently tested to confirm that they did not have FXS. Exclusion criteria for both groups included the presence of sensory impairments, or any other serious medical or neurological condition that affected growth or development (e.g., seizure disorder, diabetes, congenital heart disease).
Fifty-six individuals with FXS (36 male, 20 female) and 20 individuals diagnosed with idiopathic developmental disability (10 male, 10 female) traveled to Stanford for the present study. However, 4 male participants with FXS were unable to comply with the study procedures, and valid eye tracking data were not available for one female participant with FXS, as well as one male control participant (see below). These individuals were excluded from the present analyses. We therefore obtained valid data for 51 individuals with FXS (32 male, 19 female) and 19 (10 female, 9 male) controls. All procedures were approved by the local IRB at Stanford University and parental consent and participant assent was obtained in all cases.
Table I shows the background characteristics for the two groups. The two groups were well matched in terms of chronological age and developmental age, as evidenced by similar mean scores obtained on the subscales and composite score of the Vineland Adaptive Behavior Scales (VABS) [Sparrow et al., 2008], a well-established measure of developmental functioning. The VABS adaptive behavior composite standard score was 58.47 (SD = 23.47) for individuals with FXS and 57.68 (SD =16.78) for controls, indicating that the level of functioning in the two groups was almost 3 standard deviations below the mean. As expected for an X-linked genetic disorder, males with FXS obtained significantly lower scores than females with FXS on all subdomains of the VABS (P’s <.001). On the Social Communication Questionnaire (SCQ) [Rutter et al., 2003], 31.8% of participants with FXS and 38.9% of controls obtained scores in the ASD range (total score > 14). The mean total score obtained on the SCQ was 11.86 (SD =7.58) for individuals with FXS and 11.50 (SD =7.36) for controls, indicating that degree of autistic symptoms in the two groups was also comparable. As expected, males with FXS obtained significantly higher total scores on the SCQ than females with FXS (P <.05).
TABLE I.
Demographic Characteristics of the Sample. Means, SD’s, and t-tests Evaluating the Difference Between Means are Shown
| Measure | Fragile X | Sig. | Controls | Sig. | Total sample | Sig. | |||
|---|---|---|---|---|---|---|---|---|---|
| Girls (N =19) | Boys (N =32) | Girls (N =10) | Boys (N =9) | Fragile X (N =51) | Controls (N =19) | ||||
| Age (years) | 20.2 (4.2) | 20.2 (3.7) | NS | 20.0 (3.3) | 18.7 (2.5) | NS | 20.2 (3.8) | 19.4 (2.9) | NS |
| VABS composite1 | 76.2 (23.6) | 48.4 (16.9) | <.001 | 61.3 (17.9) | 53.7 (15.5) | NS | 58.5 (23.6) | 57.7 (16.8) | NS |
| Communication | 74.6 (21.8) | 38.5 (17.8) | <.001 | 58.7 (18.7) | 46.6 (14.6) | NS | 52.0 (26.1) | 53.0 (17.6) | NS |
| Daily Living | 82.3 (24.7) | 53.5 (23.8) | <.001 | 71.2 (22.9) | 64.0 (21.4) | NS | 64.3 (27.8) | 67.8 (21.9) | NS |
| Socialization | 77.0 (22.1) | 59.2 (21.4) | <.01 | 67.5 (19.6) | 64.0 (19.0) | NS | 65.9 (23.1) | 65.8 (18.8) | NS |
| Autistic symptoms2 | 9.1 (7.3) | 13.8 (7.3) | <.05 | 12.0 (7.7) | 11.0 (7.5) | NS | 11.9 (7.6) | 11.50 (7.4) | NS |
Vineland Adaptive Behavior Scales [Sparrow et al., 2008]
Social Communication Questionnaire [Rutter et al., 2003]
Apparatus
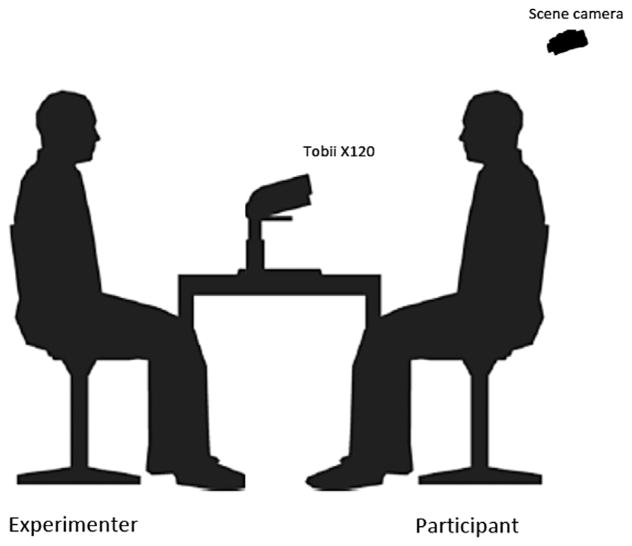
A Tobii X120 Eye Tracker (Tobii Technology) was used to collect all eye tracking data in the study. The Tobii X120 is a stand-alone eye tracking device that can be customized for various experimental settings. The Tobii X120 was particularly suited for the present study as it allows large freedom of head movement, thus ensuring that participants could behave as naturally as possible during the experiment without distraction from intrusive machinery. The X120 was also advantageous because no device or headgear was required to be attached to the participant during the experiment. The Tobii X120 has a data rate of 120 Hz, a latency of 35 ms, a mean time to tracking recovery of 100 ms, an accuracy of 0.5°, a spatial resolution of .2 degrees, a drift of 0.3°, a freedom-of-head movement of 30 × 22 × 30 cm, a tracker field of view of 22 × 22 × 30 cm and a top head-motion speed of 35 cm/sec. A custom setup was employed for the present study to ensure that the experimental setting was as naturalistic as possible. The Tobii X120 was positioned on a height-adjustable rectangular table (60 cm by 90 cm) with two height-adjustable chairs (one for the participant, one for the experimenter) positioned either side of the table (see Fig. 1).
FIG. 1.
Customized configuration of the Tobii X120 eye-tracker.
When seated in the height-adjustable chairs, the distance between the experimenter and participant was approximately, 1 m. A scene camera (Sony Handycam DCR-HC37E), positioned directly above and behind the participant’s chair, was used to monitor the face of the experimenter. A mini user camera (Microsoft LifeCam VX-5000), positioned centrally on top of the eye tracker, was used to simultaneously monitor the face of the participant. In order to calibrate the eye tracker to the area where the participant would be looking during the experiment, a calibration board (60 cm by 60 cm) was mounted perpendicular to the left edge of the table by a moveable computer monitor arm so that the center of the board was in the same plane as to where the experimenter’s face would be positioned (see Fig. 1). The calibration board had four small magnetic orange-colored circles (diameter =2 cm) arranged in a square grid (27 cm by 27 cm). The distance between the participant’s eyes and the eye tracker was approximately 70 cm and the gaze angle was 20°. All eye movement and video/audio data were collected on a Dell Precision M6300 laptop computer using Tobii Studio 1.7.2 software.
Procedure
Participants were instructed to sit down in the appropriate chair and to remain seated throughout the experiment. Depending on the height of the participant, the height of the chair was adjusted until the participant could see all four points on the calibration board. In order to calibrate the eye tracker to the area where the experimenter’s face would be situated, the participant was instructed to look at the top left and bottom right corners of the grid on the calibration board in quick succession. The calibration process took approximately, 2–10 min depending on the individuals’ compliance level. To achieve calibration, the experimenter worked with the individual to simplify instruction, pointing to the dots if necessary, and encouraging the participant to persist with calibration. No additional training was needed for the participants to complete the task. Four low functioning males with FXS were unable to comply with the calibration procedure due to an inability to direct attention to the calibration board for a sufficient length of time to enable tracking of the eyes, and these participants were not included in the study. Once the calibration was successful, the calibration board was moved aside and the experimenter sat in the chair opposite the participant with her face positioned in the same plane as to where the calibration board had been positioned (see Fig. 1). The experimenter then looked directly at the participant, and said, “OK, now we are going to have a conversation and I need you to look me in the eyes as much as possible while we are talking.” The experimenter then proceeded to engage the participant in simple conversation, asking about familiar topics such as the participant’s family, friends, and hobbies. The experimenter kept as still as possible (to avoid moving outside of the range of the eye tracker), and looked directly at the participant at all times, but in all other aspects acted as naturally as possible. If the participant did not make eye contact for 30-s, the experimenter delivered an eye contact “prompt” (i.e., reminding the participant to make eye contact) and encouraged the participant to continue looking. Each conversation lasted ten minutes.
Training of the experimenter involved a thorough review of written training protocols (i.e., introduction wording, eye contact prompting details, conversation topics), and several practice sessions. Due to the specific physical and behavioral phenotype of FXS, the experimenter could not be blinded to group assignment. However, the experimenter performed the task in a similar way across all participants using consistent language when prompting for eye contact and initiating conversation topics. Conversation was allowed to flow and follow the participants’ interests to promote a naturalistic setting. To check the consistency of experimenter behavior across subjects, we coded the amount of time that the experimenter talked to the participant (including the administration of eye contact prompts) for a representative sample of participants (10 males with FXS, 11 females with FXS and 6 controls). The mean percentage of experimenter talking was 48.1% (SD = 15.9%) for females with FXS, 57.5% (SD = 5.0%) for males with FXS and 50.7% (SD = 11.0%) for controls.
Data Analysis
The goal of our analysis was to classify whether and when participants fixated on the experimenter’s face, and which specific subregions of the face were fixated. Because the experimenter exhibited natural head movements during the conversation flow, we first used an automatic face detection algorithm to identify regions of interest (ROIs) in the scene camera and then calculated the proportion of fixations within these ROIs.
Face detection was accomplished using a variant of the deformable part model (DPM) [Zhu and Ramanan, 2012] which applied a template to each image independently; this template was composed of a number of parts whose relationship to one another is elastic, and so permits deformation caused by differences in face structure, facial expression, or pose. The DPM algorithm allows the detection of faces and face structure even when faces are at non-canonical angles (e.g. profile) or are partially occluded. Because the scene camera videos contained face-forward images on a blank background (see Fig. 2 for an example video frame), the algorithm’s performance was essentially perfect. To increase computational efficiency, we sub-sampled each video to 5 frames per second and applied DPM to each. The result from our algorithm was a map of keypoints on the face for each frame, which we verified by hand through spot-checks of each participant’s video.
FIG. 2.

Example frame from the scene camera video. The red dot shows the participant’s point of gaze.
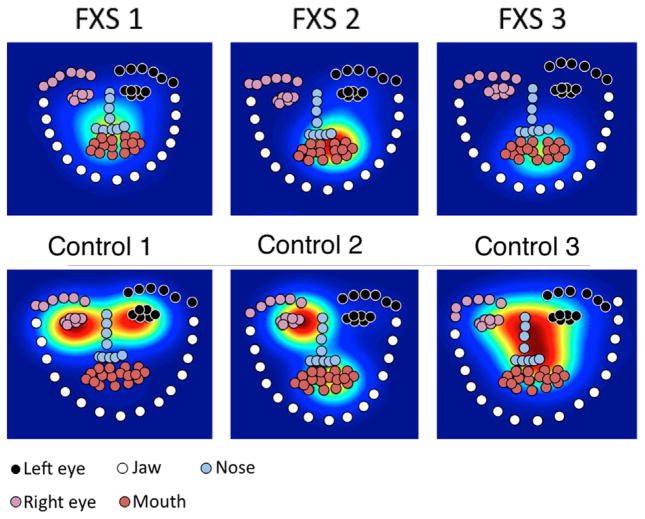
We next grouped keypoints into a set of ROIs (left eye, right eye, nose, mouth, and jaw; see Fig. 3). To map eye-movements to these ROIs, we wrote custom Matlab scripts, based on those described in Frank, Vul, and Saxe (2012). These scripts processed the raw tabular data output from the Tobii tracker, averaging gaze position across eyes. We then computed the ROI into which each point of gaze fell by associating each point with the closest keypoint, up to a threshold of 20 pixels.
FIG. 3.
Sample heatmaps showing density of fixation (warmer colors show more fixation) for representative FXS and control participants. Circles show grouped keypoints for the region-of-interest analyses.
For each participant, we calculated dwell time in each ROI as a proportion of total looking time (excluding looks away and other missing data). Total looking at the face was computed as the sum of looking times to each ROI. We also calculated average episode length, a measure of the average length of gaze at the face, by smoothing across looks away less than 600 msec and then computed the average length of a period of looking at the face. The goal of this smoothing was to avoid taking into account short losses of tracking data and extremely brief (one fixation) looks away from the face. For statistical analyses, we used a log transformation to improve the normality of the data distributions. There were no laterality effects, and so the left and right eye regions were combined into one region. Overall, we were able to include data from 87% of frames from FXS participants and 92% of frames from controls.
RESULTS
Table II shows a summary of the results comparing the various metrics of social gaze across groups.
TABLE II.
Gaze Metrics for Each Group. Means, SD’s, and t-tests Evaluating the Difference Between Means are Shown
| Metric | Fragile X | Sig. | Controls | Sig. | Total sample | Sig. | |||
|---|---|---|---|---|---|---|---|---|---|
| Girls (N =19) | Boys (N =32) | Girls (N =10) | Boys (N =9) | Fragile X (N =51) | Controls (N =19) | ||||
| Proportion of gaze | |||||||||
| Face | .33 (.25) | .12 (.16) | <.001 | .53 (.25) | .57 (.28) | NS | .20 (.22) | .55 (.26) | <.001 |
| Eyes | .16 (.20) | .03 (.07) | <.001 | .26 (.26) | .23 (.23) | NS | .08 (.15) | .24 (.24) | <.001 |
| Nose | .09 (.11) | .03 (.055) | <.01 | .13 (.15) | .20 (.16) | NS | .05 (.08) | .16 (.16) | <.000 |
| Mouth | .06 (.08) | .05 (.10) | NS | .07 (.06) | .11 (.12) | NS | .06 (.09) | .09 (.09) | <.05 |
| Jaw | .01 (.01) | .01 (.01) | NS | .06 (.14) | .03 (.03) | NS | .01 (.02) | .04 (.10) | NS |
| Face looking | |||||||||
| Episode length (s) | 1.8 (1.1) | 1.3 (0.5) | <.05 | 3.8 (2.1) | 4.7 (2.8) | NS | 1.5 (0.8) | 4.2 (2.4) | <.001 |
| Inter-episode length (s) | 3.0 (1.1) | 5.6 (2.2) | <.001 | 2.2 (0.3) | 2.7 (2.1) | NS | 4.6 (2.2) | 2.4 (1.4) | <.001 |
Proportion of Social Gaze
Our primary metric was the proportion of looking time to the experimenter’s face. The data show that participants with FXS spent significantly less proportion of time looking at the experimenter’s face (M =.20, SD =.22) compared to controls (M =.55, SD =.26) (t(68) =5.58, P <.001). As expected, males with FXS spent significantly less time looking at the experimenter’s face (M =.12, SD =.16) then females with FXS (M =.33, SD =.25) (t(49) =3.69, P <.001).
Data concerning the proportion of looking time at the various regions of the experimenter’s face are also shown in Table II. Here, participants with FXS spent significantly less proportion of time compared to controls looking at the eyes (FXS: M =.08; controls: M =.24), nose (FXS: M =.05; controls: M =.16) and mouth (FXS: M =.01; controls: M =.04) (all P’s <.05). There was no difference between the groups in terms of the proportion of looking time to the jaw. Males with FXS spent significantly less time looking at the eyes (P <.001) and nose (P <.05) compared to females with FXS.
Given that overall looking time to the face was significantly lower in participants with FXS, we examined whether differences in looking times to the various regions of the face would remain when overall looking time to the face was controlled statistically in the analysis. When total looking time to the face was controlled, there were no differences between the groups in terms of where participants looked at the face (all P’s >.05).
Episode Length of Social Gaze
Table II shows the mean episode (and inter-episode) length of social gaze to the face for the two groups. Statistical analysis indicated that participants with FXS displayed significantly shorter episodes of face looking (M =1.5 s, SD =0.8 s) compared to controls (M =4.2 s, SD =2.4 s), (t(68) =7.07, P <.001). The mean inter-episode length of face looking was also significantly longer for participants with FXS (M =4.6 s, SD =2.2) compared to controls (M =2.4 s, SD =1.4) (t(68) =4.04, P <.001). Within the FXS group, as expected, males with FXS had significantly shorter episode lengths (M =1.3 s, SD =0.5) than females with FXS (M =1.8 s, SD =1.1) (t(49) =2.61, P =.012) and also had significantly longer inter-episode lengths (M =5.6 s, SD =2.2) than females with FXS (M =3.0 s, SD =1.1) (t(49) =4.95, P <.001).
Regression Analyses
To examine how participant age, communication ability, and degree of autistic symptoms affected levels of social gaze, we conducted a multiple regression analysis of the data in each group. The independent variables for each analysis were participant age, the Communication domain standard score on the VABS-II, and the total score on the SCQ. The dependent variable for each analysis was the proportion of gaze to the face. Table III shows the results.
TABLE III.
Results of the Multiple Regression Analysis Predicting Social Gaze in Each Group
| Group | Independent variables | Beta | P |
|---|---|---|---|
| FXS (N =51) | Age | .116 | NS |
| Communication | .483 | .005 | |
| Autistic symptoms | .047 | NS | |
| Controls (N =19) | Age | .155 | NS |
| Communication | −.107 | NS | |
| Autistic symptoms | −.795 | <.001 |
Beta is the standardized regression coefficient.
In the FXS group, there was a significant effect of communication ability on levels of social gaze (β =.483, P =.005), but no effect of degree of autistic symptoms (β =−.047, P >.05) or age (β =.0116, P >.05). These data indicted that increased communication ability predicted increased levels of social gaze in individuals with FXS when age and degree of autistic symptoms were controlled. In the control group, there was a significant effect of degree of autistic symptoms on levels of social gaze (β =−.795, P <.001), but no effect of age (β =.155, P >.05) or communication ability (β =−.107, P <.05). These data indicated that increased autistic symptoms predicted lower levels of social gaze in controls when age and communication ability were controlled.
DISCUSSION
Previous investigations employing eye tracking methodology to quantify social gaze in individuals with FXS have been limited by poor ecological validity. For example, the literature is replete with studies in which participants have been required to passively view social scenes on video or to view static images of faces [Cornish et al., 2007; Dalton et al., 2008; Holsen et al., 2008; Farzin et al., 2009; Shaw and Porter, 2013; Williams et al., 2013]. In these studies, we believe that a crucial aspect of the social milieu is missing –namely the back-and-forth interaction with a live person. We therefore developed a relatively unobtrusive method to track eye gaze during a real-life conversation using existing eye tracking software. We believe that an advantage of this method is that more a valid representation of gaze patterns can be obtained.
The experimental social setting was designed so that participants could interact directly with a social partner while social gaze behavior was unobtrusively measured on a Tobii X120 eye tracker. An advantage of this system is that it is stand-alone and does not require any wires or intrusive machinery to be worn on the body. The eye tracker was simply placed on the table in front of the participant, and participants could move their head freely, as would naturally occur during a social interaction. Using this naturalistic social paradigm, we were able to obtain valid eye tracking data on 91% of participants with FXS, aged 12–28 years. However, four low functioning males with FXS were unable to follow the calibration procedures indicating that there may be IQ limitations using this paradigm. For the remaining participants, the percentage of frames that needed to be discarded due to eye tracking errors was relatively low (13%, see above). These data suggest that valid eye tracking data can be collected for the majority of individuals with FXS in a naturalistic social setting.
Overall, our results showed that individuals with FXS engaged in social gaze approximately 20% of the time on average during the 10-min social interaction. These data are similar to the levels of social gaze obtained in previous eye-tracking studies of individuals with FXS that employed static photographs of faces [Farzin et al., 2009], as well as in studies in which eye gaze has been manually coded from video recordings during live social interactions with others [Cohen et al., 1989, 1991; Hall et al., 2006, 2009]. In our ROI analyses, we found that on average, individuals with FXS looked at the eye region for about 8% of the time, at the nose region for about 6% of the time, and at the mouth region for about 6% of the time. By contrast, control individuals looked at the eye region for about 25% of the time, at the nose region for about 16% of the time, and at the mouth region for about 9% of the time on average. Individuals with FXS therefore appeared to be particularly impaired in looking at the eyes and the nose regions relative to controls. However, when overall looking time to the face was controlled statistically, there were no differences between the groups in terms of where participants looked. These data indicate that individuals with FXS exhibited less attentiveness to the face overall.
We compared individuals with FXS to a group of individuals who were matched on age, developmental age and autistic symptomatology, as opposed to a group of typically developing individuals, for several reasons. First, we wanted to determine whether impairments in social gaze were specific to FXS, as opposed to being simply associated with impairments shown by individuals with developmental disabilities in general. Several previous studies of social gaze have compared individuals with FXS to typically developing individuals, and not surprisingly, have found that individuals with FXS show significant impairments in social gaze relative to that group. However, given that these groups differ significantly in level of functioning and degree of social impairments, the more interesting question is whether social gaze impairments in FXS could be detected relative to individuals with similar levels of social impairment but who did not have FXS (see Cohen et al., 1989, 1991]. We found that episode lengths of social gaze were almost 3 times shorter (1.5 s vs. 4.2 s on average) and mean inter-episode lengths of social gaze were almost twice as long (4.6 s vs. 2.4 s) in individuals with FXS compared to controls. Taken together, our data indicate that impairments in social gaze behavior in FXS are present even relative to individuals with similar levels of developmental disability and autistic symptoms [c.f., Cohen et al., 1991].
In our regression analyses, we examined how participant age, communication ability, and degree of autistic symptoms affected social gaze levels in each group. In the FXS group, we found that higher levels of communication ability were associated with higher levels of social gaze, supporting the findings of Cohen and colleagues [Cohen et al., 1991] who also found that eye gaze levels improved with increases in communicative ability. Interestingly, in the control group, we found that higher levels of autistic symptomatology were associated with greater impairments in social gaze. Although this analysis may have been affected by the small sample size, these data provide external validity to our findings, given that individuals with a greater degree of autistic symptoms should, by definition, be more likely to show more social impairments. It is unclear why this relationship was not found in the FXS group. One potential explanation could be that autistic-like symptoms are qualitatively different in individuals diagnosed with FXS compared to those diagnosed with autism [see Cohen, 1996; Hall et al., 2010]. Thus, although the groups were well matched on the SCQ, it seems likely that the symptoms listed on the SCQ may not adequately capture the nature of the social impairment displayed by individuals with FXS. For example, many items on the SCQ are related to social indifference, yet many individuals with FXS actually appear interested in interacting socially with others, but are subsequently hampered by increased levels of physiological arousal once the interaction begins [Hall et al., 2009]. It is possible, therefore, that physiological activity may have been significantly increased in individuals with FXS compared to controls during the social interaction. In future studies, we plan to include simultaneous monitoring of physiological activity during the social interaction paradigm to test this hypothesis [see Hall et al., 2009].
There are several limitations of the study that should be mentioned. First, given that a typically developing group of individuals was not included in the study, it is unknown how levels of gaze behavior would be affected under these conditions in individuals without a developmental disability. In a previous study that employed a similar face-to-face paradigm in which gaze duration was coded from videotape rather than on an eye-tracker, we found that social gaze to the face in typically developing siblings of children with FXS was typically quite high (occurring > 90% of the time) [Hall et al., 2009]. This is most likely because typically developing individuals in this age range may have encountered similar social situations in the past (e.g., interviews, one-to-one discussions with parents or teachers) and were more able to comply with simple instructions such as “please look at me as much as possible”. Future studies need to establish normative data on social gaze in younger populations and in cases where no explicit instruction is given. A second limitation concerns the fact that the conversation between the participant and the experimenter itself was somewhat contrived. The experimenter was instructed to engage the participant in a conversation while looking at the participant 100% of the time. In the studies conducted by Cohen and colleagues, mothers were observed interacting naturalistically with their children via a one-way mirror, and trained observers coded dyadic gaze patterns (mother looking at child, child looking at mother, both looking at each other, or neither looking). Given that the experimenter never looked away during the experiment, it is unknown whether increased levels of eye gaze would have occurred once the experimenter looked away [c.f. Cohen et al., 1991]. It would also be interesting to determine whether the amount of talking may have influenced the amount of eye gaze. It seems likely, for example, that social gaze may decrease significantly when the child is speaking as compared to when the child is listening. A final limitation concerns whether there may have been a bias towards inclusion of higher functioning individuals with FXS simply because we included individuals who could travel to Stanford for a 3-day visit and were able to sit in a chair for 10 min without attempting to leave the room.
In summary, the present study provides evidence to suggest that social gaze can be measured objectively during a naturalistic social paradigm in individuals with FXS. Over the past decade, several Phase II clinical trials have been conducted to test the efficacy of FXS-specific pharmacological interventions but these studies have been limited by the dearth of reliable, valid, sensitive and objective outcome measures available to evaluate outcomes in individuals with developmental disabilities [Berry-Kravis et al., 2013]. Given that social gaze deficits are so prevalent in individuals with FXS, the paradigm described here may provide a useful tool to meet the requirements for an ecologically valid outcome measure to evaluate social impairments in FXS in clinical trials.
Acknowledgments
Grant sponsor: NIH grants; Grant numbers: MH050047, MH081998.
We thank the families for participating in this study, Hans Bergman for helping to set up the customized configuration, and Mai Manchanda and Brigid McCarthy for helping with the project. Dr. Reiss has received consultancy fees from Novartis and Genentech. Dr. Farzin is now at Lumos Labs Inc., San Francisco.
References
- Bennetto L, Pennington BF, Porter D, Taylor AK, Hagerman RJ. Profile of cognitive functioning in women with the fragile X mutation. Neuropsychology. 2001;15:290–299. [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Abbeduto L, Reiss AL, Beckel-Mitchener A, Urv TK. Outcome measures for clinical trials in fragile X syndrome. J Dev Behav Pediatr. 2013;34:508–522. doi: 10.1097/DBP.0b013e31829d1f20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. The development of gaze following and its relation to language. Dev Sci. 2005;8:535–543. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL. A theoretical analysis of the role of hyperarousal in the behavior and learning of fragile X males. Ment Retard Dev Disabil Res Rev. 1996;1:286–291. [Google Scholar]
- Cohen IL, Fisch GS, Sudhalter V, Wolf-Schein EG, Hanson D, Hagerman R, Jenkins EC, Brown WT. Social gaze, social avoidance, and repetitive behavior in fragile X males: A controlled study. Am J Ment Retard. 1988;92:436–446. [PubMed] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Parent-child dyadic gaze patterns in fragile X males and in non-fragile X males with autistic disorder. J Child Psychol Psychiatry. 1989;30:845–856. doi: 10.1111/j.1469-7610.1989.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Effects of age and communication level on eye contact in fragile X males and non-fragile X autistic males. Am J Med Genet. 1991;38:498–502. doi: 10.1002/ajmg.1320380271. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Scerif G, Karmiloff-Smith A. Tracing syndrome-specific trajectories of attention across the lifespan. Cortex. 2007a;43:672–685. doi: 10.1016/s0010-9452(08)70497-0. [DOI] [PubMed] [Google Scholar]
- Cornish K, Turk J, Levitas A. Fragile X syndrome and autism: Common developmental pathways? Curr Pediat Rev. 2007b;3:61–68. [Google Scholar]
- Cornish K, Turk J, Hagerman R. The fragile X continuum: New advances and perspectives. J Intellect Disabil Res. 2008;52:469–482. doi: 10.1111/j.1365-2788.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Turk J, Wilding J, Sudhalter V, Munir F, Kooy F, Hagerman R. Annotation: Deconstructing the attention deficit in fragile X syndrome: A developmental neuropsychological approach. J Child Psychol Psychiatry. 2004;45:1042–1053. doi: 10.1111/j.1469-7610.2004.t01-1-00297.x. [DOI] [PubMed] [Google Scholar]
- Csibra G, Gergely G. Social learning and social cognition: The case for pedagogy. In: Munakata Y, Johnson MH, editors. Processes of Change in the Brain and Cognitive Development. Attention and Performance. XXI. Oxford: Oxford University Press; 2006. pp. 249–274. [Google Scholar]
- Dalton KM, Holsen L, Abbeduto L, Davidson RJ. Brain function and gaze fixation during facial-emotion processing in fragile X and autism. Autism Res. 2008;1:231–239. doi: 10.1002/aur.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake C, Bui Q, Bulhak-Paterson D, Huggins R, Loesch DZ. Behavioural and cognitive phenotypes in idiopathic autism versus autism associated with fragile X syndrome. J Child Psychol Psychiatry. 2009;50:290–299. doi: 10.1111/j.1469-7610.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- Doherty-Sneddon G, Whittle L, Riby DM. Gaze aversion during social style interactions in autism spectrum disorder and Williams syndrome. Res Dev Disabil. 2013;34:616–626. doi: 10.1016/j.ridd.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: The neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proc Natl Acad Sci USA. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Rivera SM, Hessl D. Brief report: Visual processing of faces in individuals with fragile X syndrome: an eye tracking study. J Autism Dev Disord. 2009;39:946–952. doi: 10.1007/s10803-009-0744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Scaggs F, Hervey C, Berry-Kravis E, Hessl D. Reliability of eye tracking and pupillometry measures in individuals with fragile X syndrome. J Autism Dev Disord. 2011;41:1515–1522. doi: 10.1007/s10803-011-1176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MC, Vul E, Saxe R. Measuring the development of social attention using free-viewing. Infancy. 2012;17:355–375. doi: 10.1111/j.1532-7078.2011.00086.x. [DOI] [PubMed] [Google Scholar]
- Frigerio E, Burt DM, Gagliardi C, Cioffi G, Martelli S, Perrett DI, Borgatti R. Is everybody always my friend? Perception of approachability in Williams syndrome. Neuropsychologia. 2006;44:254–259. doi: 10.1016/j.neuropsychologia.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci USA. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. J Med Genet. 2008;45:498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Debernardis GM, Reiss AL. Social escape behaviors in children with fragile X syndrome. J Autism Dev Disord. 2006;36:935–947. doi: 10.1007/s10803-006-0132-z. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in children and adolescents with fragile X syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48:320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Hirt M, Rezvani A, Reiss AL. Autism in fragile X syndrome: A category mistake? J Am Acad Child Adolesc Psychiatry. 2010;49:921–933. doi: 10.1016/j.jaac.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Dalton KM, Johnstone T, Davidson RJ. Prefrontal social cognition network dysfunction underlying face encoding and social anxiety in fragile X syndrome. Neuroimage. 2008;43:592–604. doi: 10.1016/j.neuroimage.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier RJ, Batty M. Neural bases of eye and gaze processing: The core of social cognition. Neurosci Biobehav Rev. 2009;33:843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Bellugi U, Lai Z, Chiles M, Reilly J, Lincoln A, Adolphs R. II. Hypersociability in Williams Syndrome. J Cogn Neurosci. 2000;12:30–46. doi: 10.1162/089892900561968. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, Cox C, Capone GT, Stanard P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. Am J Med Genet A. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kennedy CH, Caruso M, Thompson T. Experimental analyses of gene-brain-behavior relations: Some notes on their application. J Appl Behav Anal. 2001;34:539–549. doi: 10.1901/jaba.2001.34-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JP, Bell J. A pedigree of mental defect showing sex-linkage. J Neurology Psychiatry. 1943;6:154–157. doi: 10.1136/jnnp.6.3-4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocco MM. Math learning disability and math LD subtypes: Evidence from studies of Turner syndrome, fragile X syndrome, and neurofibromatosis type 1. J Learn Disabil. 2001;34:520–533. doi: 10.1177/002221940103400605. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Singh Bhatia N, Lesniak-Karpiak K. Visuospatial skills and their association with math performance in girls with fragile X or Turner syndrome. Child Neuropsychol. 2006;12:87–110. doi: 10.1080/09297040500266951. [DOI] [PubMed] [Google Scholar]
- Morales M, Mundy P, Delgado CEF, Yale M, Neal R, Schwartz HK. Gaze following, temperament, and language development in 6-month-olds: A replication and extension. Infant Behav Dev. 2000;23:231–236. [Google Scholar]
- Murphy MM. A review of mathematical learning disabilities in children with fragile X syndrome. Dev Disabil Res Rev. 2009;15:21–27. doi: 10.1002/ddrr.49. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: Analyzing gene-brain-behavior relationships in child developmental psychopathologies. Dev Psychopathol. 2003;15:927–968. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- Riby DM, Doherty-Sneddon G, Whittle L. Face-to-face interference in typical and atypical development. Dev Sci. 2012;15:281–291. doi: 10.1111/j.1467-7687.2011.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionaire. Manual. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Schroeder SR, Oster-Granite ML, Thompson T. Self-injurious Behavior: Gene-Brain-Behavior Relationships. Washington, DC: American Psychological Association; 2001. [Google Scholar]
- Senju A, Johnson MH. The eye contact effect: Mechanisms and development. Trends Cogn Sci. 2009;13:127–134. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Shaw TA, Porter MA. Emotion recognition and visual-scan paths in fragile X syndrome. J Autism Dev Disord. 2013;43:1119–1139. doi: 10.1007/s10803-012-1654-1. [DOI] [PubMed] [Google Scholar]
- Skinner M, Hooper S, Hatton DD, Roberts J, Mirrett P, Schaaf J, Sullivan K, Wheeler A, Bailey DB., Jr Mapping nonverbal IQ in young boys with fragile X syndrome. Am J Med Genet A. 2005;132A:25–32. doi: 10.1002/ajmg.a.30353. [DOI] [PubMed] [Google Scholar]
- Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci. 2010;30:16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. Circle Pines, MN: AGS Publishing; 2008. [Google Scholar]
- Sudhalter V, Cohen IL, Silverman W, Wolf-Schein EG. Conversational analyses of males with fragile X, Down syndrome, and autism: Comparison of the emergence of deviant language. Am J Ment Retard. 1990;94:431–441. [PubMed] [Google Scholar]
- Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P, Bailey D., Jr ADHD symptoms in children with FXS. Am J Med Genet A. 2006;140:2275–2288. doi: 10.1002/ajmg.a.31388. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton DD, Hammer J, Sideris J, Hooper S, Ornstein PA, Bailey DB., Jr Sustained attention and response inhibition in boys with fragile X syndrome: Measures of continuous performance. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:517–532. doi: 10.1002/ajmg.b.30504. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Williams TA, Porter MA, Landon R. Viewing social scenes: A visual scan-path study comparing fragile X syndrome and Williams syndrome. J Autism Dev Disord. 2013;43:1880–1894. doi: 10.1007/s10803-012-1737-z. [DOI] [PubMed] [Google Scholar]
- Zhu X, Ramanan D. Computer Vision and Pattern Recognition (CVPR) Providence, Rhode Island: 2012. Face detection, pose estimation, and landmark localization in the wild; pp. 2879–2886. [Google Scholar]