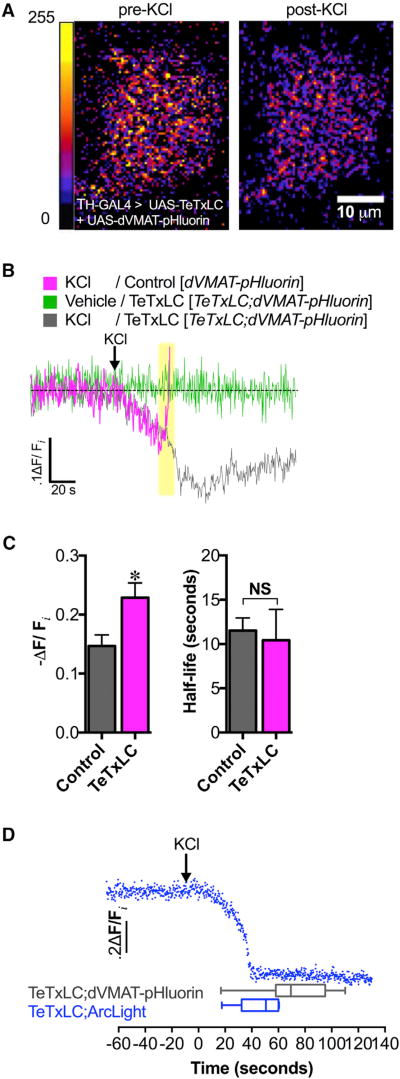
Figure 2. Depolarization-Induced Increase in Vesicular Acidification Is Independent of Exocytosis.
(A) High K+ stimulation (40 mM KCl) caused increased SV acidification in DA nerve terminals as indicated by the decrease in dVMAT-pHluorin fluorescence (right) compared to pre-treatment (left) (10 µm scale bar; false color, arbitrary fluorescence intensity units). We show a representative single-plane image of DA nerve terminals in the MV-MB1 region in adult fly brains selectively expressing dVMAT-pHluorin and TeTxLC.
(B) Averaged dVMAT-pHluorin fluorescence traces measuring depolarization-induced changes in vesicular pH in TeTxLC-expressing DA terminals (gray trace) versus control DA terminals that do not express TeTxLC (magenta trace). DA terminal-selective expression of TeTxLC eliminated the rapid dVMAT-pHluorin brightening associated with vesicle exocytosis in response to KCl (highlighted in yellow); vehicle treatment did not cause fluorescence changes (green trace).
(C) Expression of TeTxLC in DA terminals significantly enhanced SV acidification during KCl-induced stimulation relative to the pre-stimulation baseline (22.9% ± 2.5% decrease in dVMAT-pHluorin fluorescence, n=3 flies; gray bar) compared to controls where TeTxLC was not expressed (14.7% ± 1.9% decrease, n = 6; magenta bar) (p=0.037). TeTxLC expression did not affect the half-life to reach maximal acidification (t1/2 = 10.4 ± 3.5 s, n = 3) compared to the non-TeTxLC control (t1/2 = 11.5 ± 1.4 s, n = 6; p > 0.05).
(D) Optical recording of membrane depolarization of DA nerve terminals selectively co-expressing TeTxLC and ArcLight (top; blue trace). KCl treatment (indicated by arrow) decreased ArcLight fluorescence, indicating membrane depolarization. KCl-induced membrane depolarization (46.1 ± 6.7 s, n = 6) preceded exocytosis-independent vesicular acidification (71.9 ± 5.9 s, n = 17; p = 0.03). Error bars, SEM. Unpaired t tests were conducted for all analyses in (C) and (D). Fly strains: Control (TH-GAL4, UAS-dVMAT-pHluorin); TeTxLC background (UAS-TeTxLC; TH-GAL4, UAS-dVMAT-pHluorin); TeTxLC/Arc-Light (UAS-TeTxLC; UAS-ArcLight/TH-GAL4).